Clinical outcomes in real-world patients with type 2 diabetes switching from first- to second-generation basal insulin analogues: Comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study
- PMID: 29938887
- PMCID: PMC6099352
- DOI: 10.1111/dom.13345
Clinical outcomes in real-world patients with type 2 diabetes switching from first- to second-generation basal insulin analogues: Comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study
Abstract
Aims: To compare clinical outcomes in patients with type 2 diabetes (T2D) switching from insulin glargine 100 units/mL (Gla-100) or insulin detemir (IDet) to insulin glargine 300 units/mL (Gla-300) or insulin degludec (IDeg).
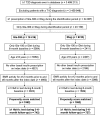
Materials and methods: We conducted a retrospective, observational study of electronic medical records for Gla-300/IDeg adult switchers (March 1, 2015 to January 31, 2017) with active records for 12-month baseline (glycated haemoglobin [HbA1c] used a 6-month baseline period) and 6-month follow-up periods. Gla-300 and IDeg switchers were propensity score-matched using baseline demographic and clinical characteristics. Outcomes were HbA1c change and goal attainment (among patients with HbA1c captured at follow-up), and hypoglycaemia with fixed follow-up (intention-to-treat [ITT]; 6 months) and variable follow-up (on-treatment [OT]; to discontinuation or 6 months).
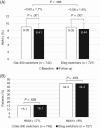
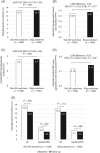
Results: Each matched cohort comprised 1592 patients. The mean decrease in HbA1c and HbA1c goal (<7.0% [53 mmol/mol] and <8.0% [64 mmol/mol]) attainment rates were similar for Gla-300 (n = 742) and IDeg (n = 727) switchers. Using fixed follow-up (ITT method), hypoglycaemia incidence decreased significantly from baseline with Gla-300 (all hypoglycaemia: 15.6% to 12.7%; P = .006; hypoglycaemia associated with inpatient/emergency department [ED] encounter: 5.3% to 3.5%; P = .007), but not with IDeg. After adjusting for baseline hypoglycaemia, no significant differences in hypoglycaemia incidence and event rate were found at follow-up (ITT) for Gla-300 vs IDeg. Using variable follow-up (OT), hypoglycaemia incidence was similar in both groups, but Gla-300 switchers had a lower inpatient/ED hypoglycaemia event rate at follow-up (adjusted rate ratio 0.56; P = .016).
Conclusions: In a real-world setting, switching from Gla-100 or IDet to Gla-300 or IDeg was associated with similar improvements in glycaemic control and hypoglycaemia in adult patients with T2D.
Keywords: 2 diabetes; basal insulin; glycaemic control; hypoglycaemia; observational study; type.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
S.D.S. has received research support from Sanofi and Novo Nordisk. T.S.B. provides research support for Abbott, Ambra, Ascensia, BD, Boehringer Ingelheim, Calibra Medical, Companion Medical, Dance Biopharm, Dexcom, Eli Lilly, Glooko, Glysens, Kowa, Lexicon, MannKind, Medtronic, Novo Nordisk, Sanofi, Senseonics, Taidoc, Versartis and Xeris, is a consultant honoraria for Abbott, Astra Zeneca, Ascensia, BD, Calibra, Capillary Biomedical, Eli Lilly, Intarcia, Medtronic, Novo Nordisk and Sanofi, and is a speaker honoraria for Abbott, Eli Lilly, Medtronic, Novo Nordisk and Sanofi. R.R. is on advisory panels for AstraZeneca, Abbvie, Sanofi, MSD, Eli Lilly, Janssen, Novo Nordisk and Physiogenex, is a speaker for Bayer and Servier, and has received research funding and provided research support to Danone Research, Amgen, Sanofi and Novo Nordisk. F.L.Z., Z.B., R.P. and J.W. are employees and stockholders of Sanofi. R.A.G. is an employee of Accenture, under contract with Sanofi. L.B. has received grants from and provided research support to AstraZeneca, Janssen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Merck & Co., Novo Nordisk and Sanofi, is a speaker for AstraZeneca, Janssen Pharmaceuticals, Inc., Merck & Co., Novo Nordisk and Sanofi, is a consultant for AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Inc., Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Novo Nordisk and Sanofi.
Figures
Similar articles
-
Comparable glycaemic control and hypoglycaemia in adults with type 2 diabetes after initiating insulin glargine 300 units/mL or insulin degludec: The DELIVER Naïve D real-world study.Diabetes Obes Metab. 2019 Sep;21(9):2123-2132. doi: 10.1111/dom.13793. Epub 2019 Jun 21. Diabetes Obes Metab. 2019. PMID: 31144445 Free PMC article.
-
Clinical outcomes in high-hypoglycaemia-risk patients with type 2 diabetes switching to insulin glargine 300 U/mL versus a first-generation basal insulin analogue in the United States : Results from the DELIVER High Risk real-world study.Endocrinol Diabetes Metab. 2022 Jan;5(1):e00306. doi: 10.1002/edm2.306. Epub 2021 Nov 22. Endocrinol Diabetes Metab. 2022. PMID: 34807513 Free PMC article.
-
Glycaemic goal attainment and hypoglycaemia outcomes in type 2 diabetes patients initiating insulin glargine 300 units/mL or 100 units/mL: Real-world results from the DELIVER Naïve cohort study.Diabetes Obes Metab. 2019 Jul;21(7):1596-1605. doi: 10.1111/dom.13693. Epub 2019 Apr 5. Diabetes Obes Metab. 2019. PMID: 30843339 Free PMC article.
-
Insulin glargine 300 units/mL for the treatment of individuals with type 2 diabetes in the real world: A review of the DELIVER programme.Diabetes Obes Metab. 2021 Aug;23(8):1713-1721. doi: 10.1111/dom.14405. Epub 2021 Jun 1. Diabetes Obes Metab. 2021. PMID: 33881797 Free PMC article. Review.
-
Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes.Diabetes Obes Metab. 2018 Mar;20(3):541-548. doi: 10.1111/dom.13105. Epub 2017 Oct 5. Diabetes Obes Metab. 2018. PMID: 28862801 Free PMC article. Review.
Cited by
-
Glycaemic control and hypoglycaemia risk with insulin glargine 300 U/mL and insulin degludec 100 U/mL in older participants in the BRIGHT trial.Diabetes Obes Metab. 2021 Jul;23(7):1588-1593. doi: 10.1111/dom.14372. Epub 2021 Apr 1. Diabetes Obes Metab. 2021. PMID: 33687748 Free PMC article.
-
Insulin degludec and insulin glargine 300 U/mL: Which of these two insulins causes less hypoglycemia?J Diabetes Investig. 2019 Nov;10(6):1595-1596. doi: 10.1111/jdi.13065. Epub 2019 May 28. J Diabetes Investig. 2019. PMID: 31135100 Free PMC article.
-
Comparable glycaemic control and hypoglycaemia in adults with type 2 diabetes after initiating insulin glargine 300 units/mL or insulin degludec: The DELIVER Naïve D real-world study.Diabetes Obes Metab. 2019 Sep;21(9):2123-2132. doi: 10.1111/dom.13793. Epub 2019 Jun 21. Diabetes Obes Metab. 2019. PMID: 31144445 Free PMC article.
-
Glycaemic control, risk of hypoglycaemia and all-cause mortality in new users of second-generation basal insulin with type 2 diabetes and chronic kidney disease: a nationwide register-based cohort study.Diabetologia. 2023 Oct;66(10):1908-1913. doi: 10.1007/s00125-023-05971-y. Epub 2023 Jul 28. Diabetologia. 2023. PMID: 37505281
-
The importance of the initial period of basal insulin titration in people with diabetes.Diabetes Obes Metab. 2020 May;22(5):722-733. doi: 10.1111/dom.13946. Epub 2020 Jan 19. Diabetes Obes Metab. 2020. PMID: 31865632 Free PMC article. Review.
References
-
- American Diabetes Association; Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(suppl 1):S1–S159. - PubMed
-
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2017 Executive Summary. Endocr Pract. 2017;23:207‐238. - PubMed
-
- Sanofi . Toujeo. https://www.drugs.com/pro/toujeo.html. Accessed February 2, 2018.
-
- Novo Nordisk . Tresiba. https://www.drugs.com/pro/tresiba.html. Accessed February 2, 2018.
-
- Clements JN, Bello L. Insulin glargine 300 units/mL: a new basal insulin product for diabetes mellitus. Am J Health Syst Pharm. 2016;73:359‐366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

