A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation
- PMID: 29939250
- PMCID: PMC6198742
- DOI: 10.1093/cvr/cvy167
A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation
Abstract
Aims: Endothelial hyperpermeability exacerbates multiple organ damage during inflammation or infection. The endothelial glycocalyx, a protective matrix covering the luminal surface of endothelial cells (ECs), undergoes enzymatic shedding during inflammation, contributing to barrier hyperpermeability. A disintegrin and metalloproteinase 15 (ADAM15) is a sheddase capable of cleaving the ectodomains of membrane-bound molecules. Herein, we tested whether and how ADAM15 is involved in glycocalyx shedding and vascular leakage during sepsis.
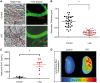
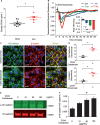
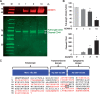
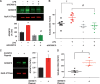
Methods and results: Dextran-150kD exclusion assay revealed lipopolysaccharide (LPS) significantly reduced glycocalyx thickness in mouse cremaster microvessels. Consistently, shedding products of glycocalyx constituents, including CD44 ectodomain, were detected with an increased plasma level after cecal ligation and puncture (CLP)-induced sepsis. The direct effects of CD44 ectodomain on endothelial barrier function were evaluated, which revealed CD44 ectodomain dose-dependently reduced transendothelial electrical resistance (TER) and caused cell-cell adherens junction disorganization. Furthermore, we examined the role of ADAM15 in CD44 cleavage and glycocalyx shedding. An in vitro cleavage assay coupled with liquid chromatography-tandem mass spectrometry confirmed ADAM15 cleaved CD44 at His235-Thr236 bond. In ECs with ADAM15 knockdown, LPS-induced CD44 cleavage and TER reduction were greatly attenuated, whereas, ADAM15 overexpression exacerbated CD44 cleavage and TER response to LPS. Consistently, ADAM15 knockout in mice attenuated CLP-induced increase in plasma CD44. Intravital and electron microscopic images revealed ADAM15 deficiency prevented LPS-induced glycocalyx injury in cremaster and pulmonary microvasculatures. Functionally, ADAM15-/- mice with better-preserved glycocalyx exhibited resistance to LPS-induced vascular leakage, as evidenced by reduced albumin extravasation in pulmonary and mesenteric vessels. Importantly, in intact, functionally vital human lungs, perfusion of LPS induced a significant up-regulation of ADAM15, accompanied by elevated CD44 in the effluent and increased vascular permeability to albumin.
Conclusion: Together, our data support the critical role of ADAM15 in mediating vascular barrier dysfunction during inflammation. Its mechanisms of action involve CD44 shedding and endothelial glycocalyx injury.
Figures


Comment in
-
ADAM-15 and glycocalyx shedding: a new perspective on sepsis-related vasomotor dysfunction.Cardiovasc Res. 2018 Nov 1;114(13):1694-1695. doi: 10.1093/cvr/cvy199. Cardiovasc Res. 2018. PMID: 30085027 No abstract available.
Similar articles
-
ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice.Am J Physiol Lung Cell Mol Physiol. 2013 Feb 1;304(3):L135-42. doi: 10.1152/ajplung.00133.2012. Epub 2012 Nov 16. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23161886 Free PMC article.
-
ADAM15 regulates endothelial permeability and neutrophil migration via Src/ERK1/2 signalling.Cardiovasc Res. 2010 Jul 15;87(2):348-55. doi: 10.1093/cvr/cvq060. Epub 2010 Feb 26. Cardiovasc Res. 2010. PMID: 20189953 Free PMC article.
-
MicroRNA-147b regulates vascular endothelial barrier function by targeting ADAM15 expression.PLoS One. 2014 Oct 15;9(10):e110286. doi: 10.1371/journal.pone.0110286. eCollection 2014. PLoS One. 2014. PMID: 25333931 Free PMC article.
-
The role of the disintegrin metalloproteinase ADAM15 in prostate cancer progression.J Cell Biochem. 2009 Apr 15;106(6):967-74. doi: 10.1002/jcb.22087. J Cell Biochem. 2009. PMID: 19229865 Review.
-
Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding.Am J Pathol. 2020 Apr;190(4):768-780. doi: 10.1016/j.ajpath.2019.11.016. Epub 2020 Feb 6. Am J Pathol. 2020. PMID: 32035885 Review.
Cited by
-
The Gut-Lung Axis in Systemic Inflammation. Role of Mesenteric Lymph as a Conduit.Am J Respir Cell Mol Biol. 2021 Jan;64(1):19-28. doi: 10.1165/rcmb.2020-0196TR. Am J Respir Cell Mol Biol. 2021. PMID: 32877613 Free PMC article. Review.
-
Protein palmitoylation regulates extracellular vesicle production and function in sepsis.J Extracell Biol. 2022 Jul;1(7):e50. doi: 10.1002/jex2.50. Epub 2022 Jul 5. J Extracell Biol. 2022. PMID: 38419739 Free PMC article.
-
Cerebrovascular glycocalyx damage and microcirculation impairment in patients with temporal lobe epilepsy.J Cereb Blood Flow Metab. 2023 Oct;43(10):1737-1751. doi: 10.1177/0271678X231179413. Epub 2023 May 26. J Cereb Blood Flow Metab. 2023. PMID: 37231664 Free PMC article.
-
Cellular Mechanisms of Lung Injury: Current Perspectives.Clin Chest Med. 2024 Dec;45(4):821-833. doi: 10.1016/j.ccm.2024.08.004. Epub 2024 Sep 20. Clin Chest Med. 2024. PMID: 39443000 Review.
-
Succinate metabolism and membrane reorganization drives the endotheliopathy and coagulopathy of traumatic hemorrhage.Sci Adv. 2023 Jun 16;9(24):eadf6600. doi: 10.1126/sciadv.adf6600. Epub 2023 Jun 14. Sci Adv. 2023. PMID: 37315138 Free PMC article.
References
-
- Rehm M, Zahler S, Lotsch M, Welsch U, Conzen P, Jacob M, Becker BF.. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 2004;100:1211–1223. - PubMed
-
- Alphonsus CS, Rodseth RN.. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014;69:777–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

