Identification of Combinations of Approved Drugs With Synergistic Activity Against Ebola Virus in Cell Cultures
- PMID: 29939303
- PMCID: PMC6249579
- DOI: 10.1093/infdis/jiy304
Identification of Combinations of Approved Drugs With Synergistic Activity Against Ebola Virus in Cell Cultures
Abstract
Background: A need to develop therapeutics to treat Ebola virus disease patients in remote and resource-challenged settings remains in the wake of the 2013-2016 epidemic in West Africa. Toward this goal, we screened drugs under consideration as treatment options and other drugs of interest, most being small molecules approved by the Food and Drug Administration. Drugs demonstrating in vitro antiviral activity were advanced for evaluation in combinations because of advantages often provided by drug cocktails.
Methods: Drugs were screened for blockade of Ebola virus infection in cultured cells. Twelve drugs were tested in all (78 pair-wise) combinations, and 3 were tested in a subset of combinations.
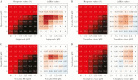
Results: Multiple synergistic drug pairs emerged, with the majority comprising 2 entry inhibitors. For the pairs of entry inhibitors studied, synergy was demonstrated at the level of virus entry into host cells. Highly synergistic pairs included aripiprazole/piperacetazine, sertraline/toremifene, sertraline/bepridil, and amodiaquine/clomiphene.
Conclusions: Our study shows the feasibility of identifying pairs of approved drugs that synergistically block Ebola virus infection in cell cultures. We discuss our findings in terms of the theoretic ability of these or alternate combinations to reach therapeutic levels. Future research will assess selected combinations in small-animal models of Ebola virus disease.
Figures
References
-
- Cross RW, Mire CE, Feldmann H, Geisbert TW. Post-exposure treatments for Ebola and Marburg virus infections. Nat Rev Drug Discov 2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

