Removing contaminants from databases of draft genomes
- PMID: 29939994
- PMCID: PMC6034898
- DOI: 10.1371/journal.pcbi.1006277
Removing contaminants from databases of draft genomes
Abstract
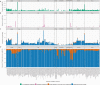
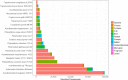
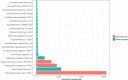
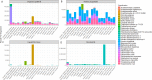
Metagenomic sequencing of patient samples is a very promising method for the diagnosis of human infections. Sequencing has the ability to capture all the DNA or RNA from pathogenic organisms in a human sample. However, complete and accurate characterization of the sequence, including identification of any pathogens, depends on the availability and quality of genomes for comparison. Thousands of genomes are now available, and as these numbers grow, the power of metagenomic sequencing for diagnosis should increase. However, recent studies have exposed the presence of contamination in published genomes, which when used for diagnosis increases the risk of falsely identifying the wrong pathogen. To address this problem, we have developed a bioinformatics system for eliminating contamination as well as low-complexity genomic sequences in the draft genomes of eukaryotic pathogens. We applied this software to identify and remove human, bacterial, archaeal, and viral sequences present in a comprehensive database of all sequenced eukaryotic pathogen genomes. We also removed low-complexity genomic sequences, another source of false positives. Using this pipeline, we have produced a database of "clean" eukaryotic pathogen genomes for use with bioinformatics classification and analysis tools. We demonstrate that when attempting to find eukaryotic pathogens in metagenomic samples, the new database provides better sensitivity than one using the original genomes while offering a dramatic reduction in false positives.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Glaser CA, Gilliam S, Schnurr D, Forghani B, Honarmand S, Khetsuriani N, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36(6):731–42. doi: 10.1086/367841 - DOI - PubMed
-
- Loman NJ, Constantinidou C, Christner M, Rohde H, Chan JZM, Quick J, et al. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli O104:H4. JAMA. 2013;309(14):1502–10. doi: 10.1001/jama.2013.3231 - DOI - PubMed
-
- Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Møller N, et al. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol. 2014;52(1):139–46. doi: 10.1128/JCM.02452-13 - DOI - PMC - PubMed
-
- Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–17. doi: 10.1056/NEJMoa1401268 - DOI - PMC - PubMed
-
- Salzberg SL, Breitwieser FP, Kumar A, Hao H, Burger P, Rodriguez FJ, et al. Next-generation sequencing in neuropathologic diagnosis of infections of the nervous system. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e251 doi: 10.1212/NXI.0000000000000251 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

