The Current Status of Drug Discovery and Development as Originated in United States Academia: The Influence of Industrial and Academic Collaboration on Drug Discovery and Development
- PMID: 29940695
- PMCID: PMC6226120
- DOI: 10.1111/cts.12577
The Current Status of Drug Discovery and Development as Originated in United States Academia: The Influence of Industrial and Academic Collaboration on Drug Discovery and Development
Abstract
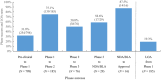
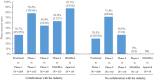
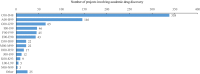
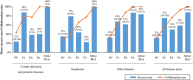
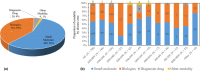
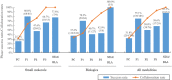
Academic drug discovery is a vital component to current drug discovery and development environments. In this study, we investigated 798 drug discovery projects that took place between 1991 and 2015 at 36 academic institutions in the United States. The observed success rates of academic drug discovery and development were 75% at phase I, 50% at phase II, 59% at phase III, and 88% at the new drug application/biologics license application (NDA/BLA) phase. These results were similar to the corresponding success rates of the pharmaceutical industry. Collaboration between academic institutions and the pharmaceutical industry seemed more important at later stages than earlier ones; all projects that succeeded at phase III or the NDA/BLA stage involved academic-industrial collaboration. Many academic research projects involved neoplasms and infectious diseases, and were focused on small molecules and biologics. The success rates and possible effects of academic-industrial collaboration seemed to vary depending on disease domains and drug modalities.
© 2018 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Figures
References
-
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 8, 959–968 (2009). - PubMed
-
- Mullard, A. 2012 FDA drug approvals. Nat. Rev. Drug Discov. 12, 87–90 (2013). - PubMed
-
- Hay, M. , Thomas, D.W. , Craighead, J.L. , Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40–51 (2014). - PubMed
-
- Biotechnology Innovation organization, Biomedtracker, Amplion Clinical Development Success Rates 2006‐2015 (BIO, Washington, DC, BioMedTracker, CA, Ampion, OR, 2016) <https://www.bio.org/sites/default/files/Clinical%20Development%20Success...>.
-
- Pharmaceutical Research and Manufacturers of America 2015 biopharmaceutical research industry profile. PhRMA, Washington, DC: (2015).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

