Effect of antiviral therapy in reducing perinatal transmission of hepatitis B virus and maternal outcomes after discontinuing them
- PMID: 29940720
- PMCID: PMC6313021
- DOI: 10.3350/cmh.2017.0082
Effect of antiviral therapy in reducing perinatal transmission of hepatitis B virus and maternal outcomes after discontinuing them
Abstract
Background/aims: There have been numerous efforts to reduce mother-to-child transmission (MTCT) of hepatitis B virus (HBV) with antiviral agents during pregnancy. However, there are limited data regarding the outcomes of pregnant women after delivery. This study was performed to evaluate the efficacy of antiviral agents in preventing MTCT of HBV and maternal long-term outcomes.
Methods: The HBV-infected pregnant women treated with antiviral agents to prevent MTCT were retrospectively reviewed. Forty-one pregnant women who received telbivudine or tenofovir during late pregnancy (28-34 week) were analyzed. Hepatitis B virus surface antibody (HBsAb) positivity was tested in 43 infants after 7 months of birth. Eleven mothers were followed >1 year after delivery.
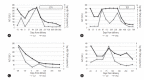
Results: The mean HBV DNA titer before antiviral therapy was 8.67 (6.60-9.49) log copies/mL, and the median age at delivery was 32 years (range, 22-40). Eleven patients were treated with tenofovir and 30 with telbivudine. The median duration was 57 days (range, 23-100), and the median HBV DNA titer at birth was 5.06 log copies/mL (range, 2.06-6.50). Antiviral treatments were associated with significant HBV DNA reduction (P<0.001). Among 43 infants (two cases of twins), HBsAb was not detected in two, subsequently confirmed to have HBV infection. Biochemical flare was observed in two of 11 mothers followed >12 months, and an antiviral agent was administered.
Conclusion: Antiviral treatment during late pregnancy effectively reduced MTCT. Long-term follow-up should be required in such cases. In addition, given that maternal biochemical flare occurred in 18% of mothers, re-administration of antiviral agents might be required.
Keywords: Antiviral agents; Hepatitis B virus; Mother-to-child transmission; Postpartum; Pregnancy.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures

Similar articles
-
Efficacy and safety of telbivudine and tenofovir disoproxil fumarate in preventing hepatitis B vertical transmission: A real-life practice.J Viral Hepat. 2019 Oct;26(10):1170-1177. doi: 10.1111/jvh.13156. Epub 2019 Jul 4. J Viral Hepat. 2019. PMID: 31177596
-
Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Jan;21(1):70-84. doi: 10.1016/S1473-3099(20)30586-7. Epub 2020 Aug 14. Lancet Infect Dis. 2021. PMID: 32805200
-
A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection.J Hepatol. 2011 Dec;55(6):1215-21. doi: 10.1016/j.jhep.2011.02.032. Epub 2011 Apr 15. J Hepatol. 2011. PMID: 21703206 Clinical Trial.
-
[Clinical study on blocking mother-to-child transmission of hepatitis B virus with high viral load and HBeAg positivity during pregnancy in Guizhou province].Zhonghua Gan Zang Bing Za Zhi. 2018 Dec 20;26(12):945-950. doi: 10.3760/cma.j.issn.1007-3418.2018.12.013. Zhonghua Gan Zang Bing Za Zhi. 2018. PMID: 30669789 Chinese.
-
Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus.Cochrane Database Syst Rev. 2023 Jun 12;6(6):CD013653. doi: 10.1002/14651858.CD013653.pub2. Cochrane Database Syst Rev. 2023. PMID: 37306558 Free PMC article. Review.
References
-
- Visvanathan K, Dusheiko G, Giles M, Wong ML, Phung N, Walker S, et al. Managing HBV in pregnancy. Prevention, prophylaxis, treatment and follow-up: position paper produced by Australian, UK and New Zealand key opinion leaders. Gut. 2016;65:340–350. - PubMed
-
- Stevens CE, Neurath RA, Beasley RP, Szmuness W. HBeAg and anti-HBe detection by radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. J Med Virol. 1979;3:237–241. - PubMed
-
- Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Motherto-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59:24–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

