Silica nanoparticles induce autophagosome accumulation via activation of the EIF2AK3 and ATF6 UPR pathways in hepatocytes
- PMID: 29940794
- PMCID: PMC6103719
- DOI: 10.1080/15548627.2018.1458174
Silica nanoparticles induce autophagosome accumulation via activation of the EIF2AK3 and ATF6 UPR pathways in hepatocytes
Abstract
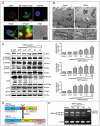
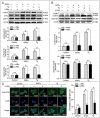
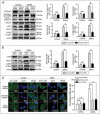
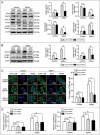
Autophagy dysfunction is a potential toxic effect of nanoparticles. Previous studies have indicated that silica nanoparticles (SiNPs) induce macroautophagy/autophagy dysfunction, while the precise mechanisms remain uncertain. Hence, the present study investigated the molecular mechanisms by which SiNPs enhanced autophagosome synthesis, which then contributed to autophagy dysfunction. First, the effects of SiNPs on autophagy and autophagic flux were verified using transmission electron microscopy, laser scanning confocal microscopy, and western blot assays. Then, the activation of endoplasmic reticular (ER) stress was validated to be through the EIF2AK3 and ATF6 UPR pathways but not the ERN1-XBP1 pathway, along with the upregulation of downstream ATF4 and DDIT3. Thereafter, the ER stress inhibitor 4-phenylbutyrate (4-PBA) was used to verify that SiNP-induced autophagy could be influenced by ER stress. Furthermore, specialized lentiviral shRNA were employed to determine that autophagy was induced via specific activation of the EIF2AK3 and ATF6 UPR pathways. Finally, the 2 autophagic genes LC3B and ATG12 were found to be transcriptionally upregulated by downstream ATF4 and DDIT3 in ER stress, which contributed to the SiNP-enhanced autophagosome synthesis. Taken together, these data suggest that SiNPs induced autophagosome accumulation via the activation of the EIF2AK3 and ATF6 UPR pathways in hepatocytes, which offers a new insight into detailed molecular mechanisms underlying SiNP-induced autophagy dysfunction, and specifically how UPR pathways regulate key autophagic genes. This work provides novel evidence for the study of toxic effects and risk assessment of SiNPs.
Keywords: ATF6; EIF2AK3; ER stress; autophagosome accumulation; autophagy dysfunction; silica nanoparticles.
Figures




Similar articles
-
Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression.Autophagy. 2014 May;10(5):766-84. doi: 10.4161/auto.27954. Epub 2014 Feb 20. Autophagy. 2014. PMID: 24589849 Free PMC article.
-
The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress.J Biol Chem. 2019 May 17;294(20):8197-8217. doi: 10.1074/jbc.RA118.002829. Epub 2019 Mar 29. J Biol Chem. 2019. PMID: 30926605 Free PMC article.
-
MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint.Autophagy. 2020 Feb;16(2):271-288. doi: 10.1080/15548627.2019.1606647. Epub 2019 Apr 21. Autophagy. 2020. PMID: 31007149 Free PMC article.
-
The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review.
-
Endoplasmic reticulum stress and the unfolded protein response: emerging regulators in progression of traumatic brain injury.Cell Death Dis. 2024 Feb 20;15(2):156. doi: 10.1038/s41419-024-06515-x. Cell Death Dis. 2024. PMID: 38378666 Free PMC article. Review.
Cited by
-
Application of Nanomaterials and Related Drug Delivery Systems in Autophagy.Molecules. 2024 Jul 26;29(15):3513. doi: 10.3390/molecules29153513. Molecules. 2024. PMID: 39124918 Free PMC article. Review.
-
Autophagy-modulating biomaterials: multifunctional weapons to promote tissue regeneration.Cell Commun Signal. 2024 Feb 15;22(1):124. doi: 10.1186/s12964-023-01346-3. Cell Commun Signal. 2024. PMID: 38360732 Free PMC article. Review.
-
Establishment and validation of individualized clinical prognostic markers for LUAD patients based on autophagy-related genes.Aging (Albany NY). 2022 Sep 29;14(18):7328-7347. doi: 10.18632/aging.204097. Epub 2022 Sep 29. Aging (Albany NY). 2022. PMID: 36178365 Free PMC article.
-
Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction.Part Fibre Toxicol. 2020 Jun 8;17(1):23. doi: 10.1186/s12989-020-00353-3. Part Fibre Toxicol. 2020. PMID: 32513195 Free PMC article.
-
Autophagic Organelles in DNA Damage Response.Front Cell Dev Biol. 2021 Apr 12;9:668735. doi: 10.3389/fcell.2021.668735. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33912571 Free PMC article. Review.
References
-
- Peynshaert K, Soenen SJ, Manshian BB, et al. Coating of quantum dots strongly defines their effect on lysosomal health and autophagy. Acta Biomater. 2017. January;15(48):195–205. PubMed PMID: 27765679. - PubMed
-
- Zhu L, Guo D, Sun L, et al. Activation of autophagy by elevated reactive oxygen species rather than released silver ions promotes cytotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in hematopoietic cells. Nanoscale. 2017. May 04;9(17):5489–5498. PubMed PMID: 28401217. - PubMed
-
- Ma X, Wu Y, Jin S, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011. November 22;5(11):8629–8639. PubMed PMID: 21974862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
