Rapid loss of early antigen-presenting activity of lymph node dendritic cells against Ag85A protein following Mycobacterium bovis BCG infection
- PMID: 29940854
- PMCID: PMC6019797
- DOI: 10.1186/s12865-018-0258-8
Rapid loss of early antigen-presenting activity of lymph node dendritic cells against Ag85A protein following Mycobacterium bovis BCG infection
Abstract
Background: Control of Mycobacterium tuberculosis (Mtb) infection requires CD4+ T-cell responses and major histocompatibility complex class II (MHC II) presentation of Mtb antigens (Ags). Dendritic cells (DCs) are the most potent of the Ag-presenting cells and are central to the initiation of T-cell immune responses. Much research has indicated that DCs play an important role in anti-mycobacterial immune responses at early infection time points, but the kinetics of Ag presentation by these cells during these events are incompletely understood.
Results: In the present study, we evaluated in vivo dynamics of early Ag presentation by murine lymph-node (LN) DCs in response to Mycobacterium bovis bacillus Calmette-Guérin (BCG) Ag85A protein. Results showed that the early Ag-presenting activity of murine DCs induced by M. bovis BCG Ag85A protein in vivo was transient, appearing at 4 h and being barely detectable at 72 h. The transcription levels of CIITA, MHC II and the expression of MHC II molecule on the cell surface increased following BCG infection. Moreover, BCG was found to survive within the inguinal LN DC pool, representing a continuing source of mycobacterial Ag85A protein, with which LN DCs formed Ag85A peptide-MHCII complexes in vivo.
Conclusions: Our results demonstrate that a decrease in Ag85A peptide production as a result of the inhibition of Ag processing to is largely responsible for the short duration of Ag presentation by LN DCs during BCG infection in vivo.
Keywords: Ag-presenting activity; Dendritic cell; In vivo; M. bovis BCG; Major histocompatibility complex class II.
Conflict of interest statement
Ethics approval and consent to participate
The mice were housed, handled, and immunized at our animal biosafety facilities, and all procedures were approved by the Institutional Animal Experimental Committee of Yangzhou University. All experiments were performed according to the national guidelines for animal welfare.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

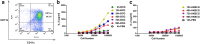
Similar articles
-
Evaluation of Immunogenicity and Protective Efficacy Elicited by Mycobacterium bovis BCG Overexpressing Ag85A Protein against Mycobacterium tuberculosis Aerosol Infection.Front Cell Infect Microbiol. 2016 Jan 28;6:3. doi: 10.3389/fcimb.2016.00003. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26858942 Free PMC article.
-
Suboptimal Antigen Presentation Contributes to Virulence of Mycobacterium tuberculosis In Vivo.J Immunol. 2016 Jan 1;196(1):357-64. doi: 10.4049/jimmunol.1501494. Epub 2015 Nov 16. J Immunol. 2016. PMID: 26573837 Free PMC article.
-
Accelerated induction of mycobacterial antigen-specific CD8+ T cells in the Mycobacterium tuberculosis-infected lung by subcutaneous vaccination with Mycobacterium bovis bacille Calmette-Guérin.Immunology. 2009 Dec;128(4):556-63. doi: 10.1111/j.1365-2567.2009.03141.x. Immunology. 2009. PMID: 19930045 Free PMC article.
-
Harnessing donor unrestricted T-cells for new vaccines against tuberculosis.Vaccine. 2019 May 21;37(23):3022-3030. doi: 10.1016/j.vaccine.2019.04.050. Epub 2019 Apr 27. Vaccine. 2019. PMID: 31040086 Free PMC article. Review.
-
Orchestration of pulmonary T cell immunity during Mycobacterium tuberculosis infection: immunity interruptus.Semin Immunol. 2014 Dec;26(6):559-77. doi: 10.1016/j.smim.2014.09.003. Epub 2014 Oct 11. Semin Immunol. 2014. PMID: 25311810 Free PMC article. Review.
Cited by
-
Can miRNA Indicate Risk of Illness after Continuous Exposure to M. tuberculosis?Int J Mol Sci. 2021 Apr 1;22(7):3674. doi: 10.3390/ijms22073674. Int J Mol Sci. 2021. PMID: 33916069 Free PMC article.
-
Activation dynamics of antigen presenting cells in vivo against Mycobacterium bovis BCG in different immunized route.BMC Immunol. 2023 Nov 27;24(1):48. doi: 10.1186/s12865-023-00589-6. BMC Immunol. 2023. PMID: 38012553 Free PMC article.
-
Discrepancy in Response of Mouse Dendritic Cells against BCG: Weak Immune Effects of Plasmacytoid Dendritic Cells Compared to Classical Dendritic Cells despite the Uptake of Bacilli.Trop Med Infect Dis. 2023 Feb 25;8(3):140. doi: 10.3390/tropicalmed8030140. Trop Med Infect Dis. 2023. PMID: 36977141 Free PMC article.
References
-
- Aguilo N, Gonzalo-Asensio J, Alvarez-Arguedas S, Marinova D, AB Gomez S, Uranga R, Spallek M, Singh M, Audran R, Spertini F, et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat Commun. 2017;8:16085. doi: 10.1038/ncomms16085. - DOI - PMC - PubMed
-
- Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, Kaufmann SH. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine's superior protection against tuberculosis. J Infect Dis. 2014;210:1928–1937. doi: 10.1093/infdis/jiu347. - DOI - PMC - PubMed
-
- Kaufmann E, Spohr C, Battenfeld S, De Paepe D, Holzhauser T, Balks E, Homolka S, Reiling N, Gilleron M, Bastian M. BCG vaccination induces robust CD4+ T cell responses to Mycobacterium tuberculosis complex-specific Lipopeptides in Guinea pigs. J Immunol. 2016;196:2723–2732. doi: 10.4049/jimmunol.1502307. - DOI - PubMed
-
- Lindestam Arlehamn CS, McKinney DM, Carpenter C, Paul S, Rozot V, Makgotlho E, Gregg Y, van Rooyen M, Ernst JD, Hatherill M, et al. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. Tuberculosis infected south Africans. PLoS Pathog. 2016;12:e1005760. doi: 10.1371/journal.ppat.1005760. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

