Outcomes and prognostic factors for aggressive posterior retinopathy of prematurity following initial treatment with intravitreal ranibizumab
- PMID: 29940900
- PMCID: PMC6019321
- DOI: 10.1186/s12886-018-0815-1
Outcomes and prognostic factors for aggressive posterior retinopathy of prematurity following initial treatment with intravitreal ranibizumab
Abstract
Background: This study sought to identify factors associated with retinal detachment and retreatment of aggressive posterior retinopathy of prematurity (APROP) initially treated with intravitreal ranibizumab (IVR) injection as well as the efficacy of IVR treatment.
Methods: This was a retrospective study. A total of 83 preterm infants (160 eyes) diagnosed with APROP who were primarily treated with IVR were included. The 160 eyes were divided into two groups based on the anatomic outcomes. Group A included 35 eyes that developed retinal detachment, and Group B included 125 eyes without retinal detachment. The following patient factors were retrospectively reviewed: gender, gestational age (GA), birth weight (BW), postmenstrual age (PMA) at first treatment, iris neovascularizations, retinal hemorrhage, neutrophil and lymphocyte counts before the first intravitreal injection, neutrophil-to-lymphocyte ratio (NLR), anatomical outcomes, additional treatment and follow-up time. Three dummy variables were created as dependent variables based on the methods of retreatment. The possible risk factors for APROP were evaluated, and statistical analyses included univariate and multivariate logistic regression.
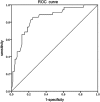
Results: A total of 160 eyes from 83 preterm infants (56 males and 27 females) underwent initial IVR treatment with a follow-up time of 17.17 ± 10.54 months. Thirty-five of the 160 (21.9%) eyes progressed to retinal detachment, and 82 of the 125 (65.6%) non-retinal detachment eyes needed retreatment, with favorable anatomical outcomes. The disease improved approximately 1.5 ± 1.2 weeks after the first IVR treatment. The mean recurrence period of APROP was approximately 7.5 ± 6.9 weeks after the first IVR treatment. Multiple logistic regression analysis revealed postmenstrual age (P < 0.001) and neutrophil count (P = 0.009) as the most significant factors for retinal detachment in APROP. Retinal hemorrhage (P = 0.007) and BW (P = 0.04) were most significantly associated with APROP recurrence and retreatment.
Conclusions: IVR injection is an effective treatment for APROP. In this study, older postmenstrual age and low neutrophil count were identified as risk factors for retinal detachment in APROP. In addition, retinal hemorrhage and low BW were significantly associated with recurrence and retreatment in non-retinal detachment APROP. Thus, patients with a lower BW, older postmenstrual age, low neutrophil count and retinal hemorrhage should be reexamined in a timely and more frequent manner.
Keywords: Aggressive posterior retinopathy of prematurity; Birthweight; Intravitreal injection; Neutrophil count; Postmenstrual age; Ranibizumab; Recurrence; Retinal detachment; Retinal hemorrhage.
Conflict of interest statement
Ethics approval and consent to participate
The study was performed in accordance with the principles of the Declaration of Helsinki, 1995 (revised in Edinburgh in 2000). This study was approved by the medical ethics committee of Peking University People’s Hospital. The parents or legal guardians of the patients provided consent for participation in the study on behalf of the underage patients, and written informed consent was obtained.
Consent for publication
Written consent for publication of the images was obtained from the patients’ guardians.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
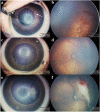
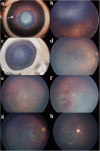
Similar articles
-
Comparison of Intravitreal Bevacizumab, Intravitreal Ranibizumab and Laser Photocoagulation for Treatment of Type 1 Retinopathy of Prematurity in Turkish Preterm Children.Curr Eye Res. 2017 Jul;42(7):1054-1058. doi: 10.1080/02713683.2016.1264607. Epub 2017 Jan 27. Curr Eye Res. 2017. PMID: 28128986
-
Comparison of Bevacizumab, Ranibizumab, and Laser Photocoagulation in the Treatment of Retinopathy of Prematurity in Turkey.Curr Eye Res. 2017 Mar;42(3):462-469. doi: 10.1080/02713683.2016.1196709. Epub 2016 Jul 15. Curr Eye Res. 2017. PMID: 27420302
-
SHORT-TERM OUTCOMES AFTER INTRAVITREAL INJECTIONS OF CONBERCEPT VERSUS RANIBIZUMAB FOR THE TREATMENT OF RETINOPATHY OF PREMATURITY.Retina. 2018 Aug;38(8):1595-1604. doi: 10.1097/IAE.0000000000001763. Retina. 2018. PMID: 28699927
-
Ranibizumab for macular edema secondary to retinal vein occlusion: a meta-analysis of dose effects and comparison with no anti-VEGF treatment.BMC Ophthalmol. 2015 Mar 29;15:31. doi: 10.1186/s12886-015-0017-z. BMC Ophthalmol. 2015. PMID: 25881069 Free PMC article. Review.
-
The efficacy and ocular safety following aflibercept, conbercept, ranibizumab, bevacizumab, and laser for retinopathy of prematurity: a systematic review and meta-analysis.Ital J Pediatr. 2023 Oct 9;49(1):136. doi: 10.1186/s13052-023-01543-3. Ital J Pediatr. 2023. PMID: 37814332 Free PMC article.
Cited by
-
The Relationship between the Aqueous VEGF Level and the Severity of Type 1 Retinopathy of Prematurity.J Clin Med. 2022 Sep 13;11(18):5361. doi: 10.3390/jcm11185361. J Clin Med. 2022. PMID: 36143009 Free PMC article.
-
Repeated intravitreal ranibizumab for reactivated retinopathy of prematurity after intravitreal ranibizumab monotherapy: vascular development analysis.Graefes Arch Clin Exp Ophthalmol. 2022 Sep;260(9):2837-2846. doi: 10.1007/s00417-022-05628-3. Epub 2022 Apr 19. Graefes Arch Clin Exp Ophthalmol. 2022. PMID: 35438363
-
Outcomes of early versus deferred laser after intravitreal ranibizumab in aggressive posterior retinopathy of prematurity.Indian J Ophthalmol. 2021 Aug;69(8):2171-2176. doi: 10.4103/ijo.IJO_3016_20. Indian J Ophthalmol. 2021. PMID: 34304203 Free PMC article. Clinical Trial.
-
High rate and large intercentre variability in retreatment of retinopathy of prematurity in infants born <24 gestational weeks.BMJ Open Ophthalmol. 2021 Apr 21;6(1):e000695. doi: 10.1136/bmjophth-2020-000695. eCollection 2021. BMJ Open Ophthalmol. 2021. PMID: 33981857 Free PMC article.
-
Evidence to date: ranibizumab and its potential in the treatment of retinopathy of prematurity.Eye Brain. 2019 Aug 23;11:25-35. doi: 10.2147/EB.S189684. eCollection 2019. Eye Brain. 2019. PMID: 31693715 Free PMC article.
References
-
- American Medical Association. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

