Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis
- PMID: 29940932
- PMCID: PMC6019800
- DOI: 10.1186/s12931-018-0831-y
Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis
Abstract
Background: Pulmonary fibrosis is a progressive and lethal disease characterized by damage to the lung parenchyma with excess extracellular matrix deposition. The involvement of endothelial cells in fibrosis development is unclear.
Methods: We isolated pulmonary endothelial cells, using a magnetic-activated cell sorting system, from mice with pulmonary fibrosis induced by intratracheal bleomycin. We characterized endothelial cells isolated at various times in the course of pulmonary fibrosis development.
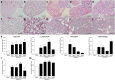
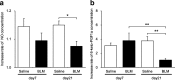
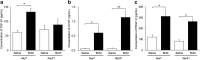
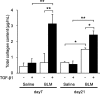
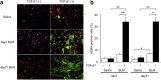
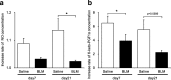
Results: Inflammatory cell infiltration was observed at 7 days after bleomycin administration, and fibrotic changes with increased collagen content were observed on day 21. Endothelial cells were isolated at these two timepoints. Levels of von Willebrand factor, plasminogen activator inhibitor-1 and matrix metalloproteinase-12 were elevated in lung endothelial cells isolated from bleomycin-treated mice at days 7 and 21. This indicated that intratracheal bleomycin administration induced endothelium injury. Expression of fibrogenic mediators, transforming growth factor (TGF)-β, connective tissue growth factor and platelet-derived growth factor-C was elevated in the cells from bleomycin-treated, compared with untreated, lungs. When endothelial cells were treated with TGF-β, α-smooth muscle actin (SMA) expression and collagen production were increased only in those cells from bleomycin-treated mouse lungs. Thapsigargin-induced prostaglandin I2 and nitric oxide production, decreased in endothelial cells from bleomycin-treated mouse lungs, compared with controls, was further suppressed by TGF-β.
Conclusion: Bleomycin administration induced functional changes in lung endothelial cells, indicating potential involvement of endothelium in pulmonary fibrogenesis.
Keywords: Bleomycin; Endothelial cell; Fibrosis; Nitric oxide; Prostaglandin I2; TGF-β; α-SMA.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Animal Care and Use Committee of Hamamatsu University School of Medicine and all experiments were performed according to guidelines of this Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
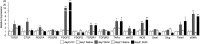
References
-
- American Thoracic S, European Respiratory S. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS executive committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. - DOI - PubMed
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

