Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages
- PMID: 29940963
- PMCID: PMC6020222
- DOI: 10.1186/s12931-018-0825-9
Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages
Abstract
Background: Airway epithelial cells and alveolar macrophages (AMs) are the first line of defense in the lung during infection. Toll-like receptor (TLR) agonists have been extensively used to define the regulation of inflammation in these cells. However, previous studies were performed in non-paired airway epithelial cells and AMs. The major goal of our study was to compare the pro- and anti-inflammatory responses of paired human primary airway epithelial cells and AMs to TLR3 and TLR4 agonists.
Methods: Tracheobronchial epithelial cells (TBEC) and AMs from four smokers and four non-smokers without lung disease were cultured with or without Poly(I:C) (PIC) (a TLR3 agonist) or LPS (a TLR4 agonist) for 4, 24 and 48 h. The immune responses of paired cells were compared.
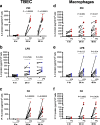
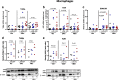
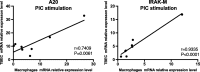
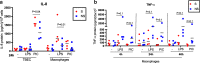
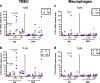
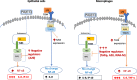
Results: TBEC and AMs showed stronger pro-inflammatory cytokine (e.g., IL-8) responses to PIC and LPS, respectively. TLR3 and TLR4 mRNA levels were similar in non-stimulated TBEC and AMs. However, PIC stimulation in AMs led to sustained up-regulation of the immune negative regulators Tollip and A20, which may render AMs less sensitive to PIC stimulation than TBEC. Unlike AMs, TBEC did not increase NF-κB activation after LPS stimulation. Interestingly, smoking status was correlated with less TLR3 and IRAK-M expression in non-stimulated TBEC, but not in AMs. PIC-stimulated TBEC and LPS-stimulated AMs from smokers vs. non-smokers produced more IL-8. Finally, we show that expression of A20 and IRAK-M is strongly correlated in the two paired cell types.
Conclusions: By using paired airway epithelial cells and AMs, this study reveals how these two critical types of lung cells respond to viral and bacterial pathogen associated molecular patterns, and provides rationale for modulating immune negative regulators to prevent excessive lung inflammation during respiratory infection.
Keywords: Alveolar macrophages; Cigarette smoke; Immune negative regulators; Inflammation; Pathogen associated molecular patterns; Toll-like receptor; Tracheobronchial epithelial cells.
Conflict of interest statement
Ethics approval and consent to participate
The study protocols were approved by the IRB at National Jewish Health.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Effects of budesonide on toll-like receptor expression in alveolar macrophages from smokers with and without COPD.Int J Chron Obstruct Pulmon Dis. 2016 May 17;11:1035-43. doi: 10.2147/COPD.S102668. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27274225 Free PMC article.
-
The effects of repeated Toll-like receptors 2 and 4 stimulation in COPD alveolar macrophages.Int J Chron Obstruct Pulmon Dis. 2018 Mar 2;13:771-780. doi: 10.2147/COPD.S97071. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29535517 Free PMC article.
-
Paralemmin-3 contributes to lipopolysaccharide-induced inflammatory response and is involved in lipopolysaccharide-Toll-like receptor-4 signaling in alveolar macrophages.Int J Mol Med. 2017 Dec;40(6):1921-1931. doi: 10.3892/ijmm.2017.3161. Epub 2017 Sep 28. Int J Mol Med. 2017. PMID: 29039447
-
Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis.Front Immunol. 2020 Oct 15;11:583042. doi: 10.3389/fimmu.2020.583042. eCollection 2020. Front Immunol. 2020. PMID: 33178214 Free PMC article. Review.
-
Innate immune mechanism in allergic asthma.Curr Allergy Asthma Rep. 2008 Sep;8(5):451-9. doi: 10.1007/s11882-008-0085-8. Curr Allergy Asthma Rep. 2008. PMID: 18682113 Review.
Cited by
-
Quercetin Preserves Oral Cavity Health by Mitigating Inflammation and Microbial Dysbiosis.Front Immunol. 2021 Nov 26;12:774273. doi: 10.3389/fimmu.2021.774273. eCollection 2021. Front Immunol. 2021. PMID: 34899728 Free PMC article.
-
Recognition of Mycobacterium tuberculosis by macrophage Toll-like receptor and its role in autophagy.Inflamm Res. 2024 May;73(5):753-770. doi: 10.1007/s00011-024-01864-x. Epub 2024 Apr 2. Inflamm Res. 2024. PMID: 38563966 Review.
-
Regulation of Host Immune Responses against Influenza A Virus Infection by Mitogen-Activated Protein Kinases (MAPKs).Microorganisms. 2020 Jul 17;8(7):1067. doi: 10.3390/microorganisms8071067. Microorganisms. 2020. PMID: 32709018 Free PMC article. Review.
-
Analysis of interleukin-1 receptor associated kinase-3 (IRAK3) function in modulating expression of inflammatory markers in cell culture models: A systematic review and meta-analysis.PLoS One. 2020 Dec 31;15(12):e0244570. doi: 10.1371/journal.pone.0244570. eCollection 2020. PLoS One. 2020. PMID: 33382782 Free PMC article.
-
Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity.Cells. 2022 Sep 25;11(19):2986. doi: 10.3390/cells11192986. Cells. 2022. PMID: 36230948 Free PMC article.
References
-
- Basset C, Holton J, O’Mahony R, Roitt I. Innate immunity and pathogen-host interaction. Vaccine. 2003;21(SUPPL. 2):S12–S23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

