Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6
- PMID: 29941022
- PMCID: PMC6019224
- DOI: 10.1186/s13287-018-0903-4
Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6
Abstract
Background: Mesenchymal stem cells (MSCs) are promising tools for the treatment of human lung disease and other pathologies relevant to newborn medicine. Recent studies have established MSC exosomes (EXO), as one of the main therapeutic vectors of MSCs in mouse models of multifactorial chronic lung disease of preterm infants, bronchopulmonary dysplasia (BPD). However, the mechanisms underlying MSC-EXO therapeutic action are not completely understood. Using a neonatal mouse model of human BPD, we evaluated the therapeutic efficiency of early gestational age (GA) human umbilical cord (hUC)-derived MSC EXO fraction and its exosomal factor, tumor necrosis factor alpha-stimulated gene-6 (TSG-6).
Methods: Conditioned media (CM) and EXO fractions were isolated from 25 and 30 weeks GA hUC-MSC cultures grown in serum-free media (SFM) for 24 h. Newborn mice were exposed to hyperoxia (> 95% oxygen) and were given intraperitoneal injections of MSC-CM or MSC-CM EXO fractions at postnatal (PN) day 2 and PN4. They were then returned to room air until PN14 (in a mouse model of severe BPD). The treatment regime was followed with (rh)TSG-6, TSG-6-neutralizing antibody (NAb), TSG-6 (si)RNA-transfected MSC-CM EXO and their appropriate controls. Echocardiography was done at PN14 followed by harvesting of lung, heart and brain for assessment of pathology parameters.
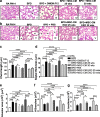
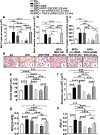
Results: Systemic administration of CM or EXO in the neonatal BPD mouse model resulted in robust improvement in lung, cardiac and brain pathology. Hyperoxia-exposed BPD mice exhibited pulmonary inflammation accompanied by alveolar-capillary leakage, increased chord length, and alveolar simplification, which was ameliorated by MSC CM/EXO treatment. Pulmonary hypertension and right ventricular hypertrophy was also corrected. Cell death in brain was decreased and the hypomyelination reversed. Importantly, we detected TSG-6, an immunomodulatory glycoprotein, in EXO. Administration of TSG-6 attenuated BPD and its associated pathologies, in lung, heart and brain. Knockdown of TSG-6 by NAb or by siRNA in EXO abrogated the therapeutic effects of EXO, suggesting TSG-6 as an important therapeutic molecule.
Conclusions: Preterm hUC-derived MSC-CM EXO alleviates hyperoxia-induced BPD and its associated pathologies, in part, via exosomal factor TSG-6. The work indicates early systemic intervention with TSG-6 as a robust option for cell-free therapy, particularly for treating BPD.
Keywords: Bronchopulmonary dysplasia; Exosomes; Hyperoxia-induced lung injury; Mesenchymal stem cells; Newborn; Pulmonary hypertension; Secretome; TSG-6; Wharton’s jelly.
Conflict of interest statement
Ethics approval
The collection of umbilical cord was approved by the Drexel University Institutional Review Board with a waiver of consent, as umbilical cords are considered discarded material. All experiments conformed to the guidelines issued by the committee on animal research of Drexel University. The study has been approved by the ethics board of Jefferson Hospital University.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures








References
-
- Reich B, Hoeber D, Bendix I, et al. Hyperoxia and the immature brain. Dev Neurosci. 2016;38:311–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

