Intrathoracic pressure regulation therapy applied to ventilated patients for treatment of compromised cerebral perfusion from brain injury
- PMID: 29941027
- PMCID: PMC6020193
- DOI: 10.1186/s13256-018-1720-1
Intrathoracic pressure regulation therapy applied to ventilated patients for treatment of compromised cerebral perfusion from brain injury
Abstract
Background: Reducing intrathoracic pressure in the setting of compromised cerebral perfusion due to acute brain injury has been associated with reduced intracranial pressure and enhanced cerebral perfusion pressure and blood flow in animals. Noninvasive active intrathoracic pressure regulation lowers intrathoracic pressure, increases preload, reduces the volume of venous blood and cerebral spinal fluid in the skull, and enhances cerebral blood flow. We examined the feasibility of active intrathoracic pressure regulation therapy in patients with brain injury. We hypothesized that active intrathoracic pressure regulation therapy would be associated with lowered intracranial pressure and increased cerebral perfusion pressure in these patients.
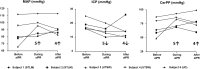
Methods: At three institutions, active intrathoracic pressure regulation therapy (CirQlator™, ZOLL) was utilized for 2 consecutive hours in five mechanically ventilated patients with brain injury. A 30-minute interval was used to collect baseline data and determine persistence of effects after device use. End-tidal carbon dioxide was controlled by respiratory rate changes during device use. The intracranial pressure, mean arterial pressure, and cerebral perfusion pressure were recorded at 5-minute intervals throughout all three periods of the protocol. Results for each interval are reported as mean and standard deviation.
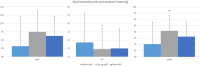
Results: Intracranial pressure was decreased in all five patients by an average of 21% during (15 ± 4 mmHg) compared to before active intrathoracic pressure regulation (19 ± 4) (p = 0.005). This effect on intracranial pressure (15 ± 6) was still present in four of the five patients 30 minutes after therapy was discontinued (p = 0.89). As a result, cerebral perfusion pressure was 16% higher during (81 ± 10) compared to before active intrathoracic pressure regulation (70 ± 14) (p = 0.04) and this effect remained present 30 minutes after therapy was discontinued. No adverse events were reported.
Conclusions: These data support the notion that active intrathoracic pressure regulation, in this limited evaluation, can successfully augment cerebral perfusion by lowering intracranial pressure and increasing mean arterial pressure in patients with mild brain injury. The measured effects were immediate on administration of the therapy and persisted to some degree after the therapy was terminated.
Keywords: Blood gas analysis; Critical care; Critical illness; Hemodynamics/physiology; Humans; Intracranial hypertension; Intracranial pressure; Life support care/methods; Nervous system diseases; Neurology; Pressure; Traumatic brain injury.
Conflict of interest statement
Ethics approval and consent to participate
Due to the nature of this study involving patients with severe BI, it was anticipated that consent decisions would be obtained via a surrogate decision maker as permitted by US laws. This study was approved by local Institutional Review Boards (IRBs) at each site: University of Texas Southwestern Medical Center IRB (042013–045), Mercy Hospital St. Louis IRB (13–021), and University of Colorado Hospital (Aurora, CO) Colorado Multiple IRB (13–2436). The information collected about study patients was kept confidential by the sponsor and by research staff at the study site in accordance with the Federal Privacy Rule. The study ClinicalTrials.gov Identifier was NCT01824576.
Competing interests
AKM, JRH, and NTB are employed by ZOLL, the manufacturer of the CirQlator device. KGL is the inventor of aIPR therapy and a consultant to ZOLL. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Intrathoracic Pressure Regulation Improves Cerebral Perfusion and Cerebral Blood Flow in a Porcine Model of Brain Injury.Shock. 2015 Aug;44 Suppl 1:96-102. doi: 10.1097/SHK.0000000000000314. Shock. 2015. PMID: 25692250
-
Comparison of Effects of Manual and Mechanical Airway Clearance Techniques on Intracranial Pressure in Patients With Severe Traumatic Brain Injury on a Ventilator: Randomized, Crossover Trial.Phys Ther. 2019 Apr 1;99(4):388-395. doi: 10.1093/ptj/pzy141. Phys Ther. 2019. PMID: 30690546 Clinical Trial.
-
Use of the intrathoracic pressure regulator to lower intracranial pressure in patients with altered intracranial elastance: a pilot study.J Neurosurg. 2013 Sep;119(3):756-9. doi: 10.3171/2013.4.JNS122489. Epub 2013 May 24. J Neurosurg. 2013. PMID: 23706051 Clinical Trial.
-
Saving the brain after mild-to-moderate traumatic injury: A report on new insights of the physiology underlying adequate maintenance of cerebral perfusion.J Trauma Acute Care Surg. 2021 Aug 1;91(2S Suppl 2):S33-S39. doi: 10.1097/TA.0000000000003286. J Trauma Acute Care Surg. 2021. PMID: 34039933 Review.
-
Treatment modalities for hypertensive patients with intracranial pathology: options and risks.Crit Care Med. 1996 Feb;24(2):311-22. doi: 10.1097/00003246-199602000-00022. Crit Care Med. 1996. PMID: 8605807 Review.
Cited by
-
Perfusion Enhancement with Respiratory Impedance After Stroke (PERI-Stroke).Neurotherapeutics. 2019 Oct;16(4):1296-1303. doi: 10.1007/s13311-019-00744-1. Neurotherapeutics. 2019. PMID: 31140115 Free PMC article.
-
Negative pressure breathing: the response of human respiration and circulation to different levels of rarefaction during inspiration.Front Physiol. 2024 Nov 27;15:1443349. doi: 10.3389/fphys.2024.1443349. eCollection 2024. Front Physiol. 2024. PMID: 39665052 Free PMC article.
References
-
- Max W, Rice DP, MacKenzie EJ. The lifetime cost of injury. Inquiry. 1990;27:332–343. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

