Regulatory Properties of the ADP-Glucose Pyrophosphorylase from the Clostridial Firmicutes Member Ruminococcus albus
- PMID: 29941423
- PMCID: PMC6088156
- DOI: 10.1128/JB.00172-18
Regulatory Properties of the ADP-Glucose Pyrophosphorylase from the Clostridial Firmicutes Member Ruminococcus albus
Abstract
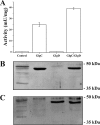
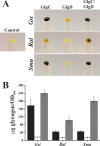
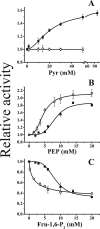
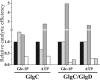
ADP-glucose pyrophosphorylase from Firmicutes is encoded by two genes (glgC and glgD) leading to a heterotetrameric protein structure, unlike those in other bacterial phyla. The enzymes from two groups of Firmicutes, Bacillales and Lactobacillales, present dissimilar kinetic and regulatory properties. Nevertheless, no ADP-glucose pyrophosphorylase from Clostridiales, the third group in Firmicutes, has been characterized. For this reason, we cloned the glgC and glgD genes from Ruminococcus albus Different quaternary forms of the enzyme (GlgC, GlgD, and GlgC/GlgD) were purified to homogeneity and their kinetic parameters were analyzed. We observed that GlgD is an inactive monomer when expressed alone but increased the catalytic efficiency of the heterotetramer (GlgC/GlgD) compared to the homotetramer (GlgC). The heterotetramer is regulated by fructose-1,6-bisphosphate, phosphoenolpyruvate, and NAD(P)H. The first characterization of the Bacillales enzyme suggested that heterotetrameric ADP-glucose pyrophosphorylases from Firmicutes were unregulated. Our results, together with data from Lactobacillales, indicate that heterotetrameric Firmicutes enzymes are mostly regulated. Thus, the ADP-glucose pyrophosphorylase from Bacillales seems to have distinctive insensitivity to regulation.IMPORTANCE The enzymes involved in glycogen synthesis from Firmicutes have been less characterized in comparison with other bacterial groups. We performed kinetic and regulatory characterization of the ADP-glucose pyrophosphorylase from Ruminococcus albus Our results showed that this protein that belongs to different groups from Firmicutes (Bacillales, Lactobacillales, and Clostridiales) presents dissimilar features. This study contributes to the understanding of how this critical enzyme for glycogen biosynthesis is regulated in the Firmicutes group, whereby we propose that these heterotetrameric enzymes, with the exception of Bacillales, are allosterically regulated. Our results provide a better understanding of the evolutionary relationship of this enzyme family in Firmicutes.
Keywords: ADP-glucose pyrophosphorylase; GlgC; GlgD; Ruminococcus albus; allosterism; fructose-1,6-bisphosphate; glycogen; glycogen metabolism; phosphoenolpyruvate; pyruvate.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Structure, function, and evolution of plant ADP-glucose pyrophosphorylase.Plant Mol Biol. 2022 Mar;108(4-5):307-323. doi: 10.1007/s11103-021-01235-8. Epub 2022 Jan 10. Plant Mol Biol. 2022. PMID: 35006475 Review.
-
The ADP-glucose pyrophosphorylase from Streptococcus mutans provides evidence for the regulation of polysaccharide biosynthesis in Firmicutes.Mol Microbiol. 2013 Dec;90(5):1011-27. doi: 10.1111/mmi.12413. Epub 2013 Oct 29. Mol Microbiol. 2013. PMID: 24112771
-
Insights into glycogen metabolism in chemolithoautotrophic bacteria from distinctive kinetic and regulatory properties of ADP-glucose pyrophosphorylase from Nitrosomonas europaea.J Bacteriol. 2012 Nov;194(22):6056-65. doi: 10.1128/JB.00810-12. Epub 2012 Sep 7. J Bacteriol. 2012. PMID: 22961847 Free PMC article.
-
Characterization of a gene cluster for glycogen biosynthesis and a heterotetrameric ADP-glucose pyrophosphorylase from Bacillus stearothermophilus.J Bacteriol. 1997 Aug;179(15):4689-98. doi: 10.1128/jb.179.15.4689-4698.1997. J Bacteriol. 1997. PMID: 9244254 Free PMC article.
-
ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis.Microbiol Mol Biol Rev. 2003 Jun;67(2):213-25, table of contents. doi: 10.1128/MMBR.67.2.213-225.2003. Microbiol Mol Biol Rev. 2003. PMID: 12794190 Free PMC article. Review.
Cited by
-
Mapping of a Regulatory Site of the Escherichia coli ADP-Glucose Pyrophosphorylase.Front Mol Biosci. 2019 Sep 25;6:89. doi: 10.3389/fmolb.2019.00089. eCollection 2019. Front Mol Biosci. 2019. PMID: 31608288 Free PMC article.
-
A critical inter-subunit interaction for the transmission of the allosteric signal in the Agrobacterium tumefaciens ADP-glucose pyrophosphorylase.Protein Sci. 2023 Sep;32(9):e4747. doi: 10.1002/pro.4747. Protein Sci. 2023. PMID: 37551561 Free PMC article.
-
Intracellular glycogen accumulation by human gut commensals as a niche adaptation trait.Gut Microbes. 2023 Jan-Dec;15(1):2235067. doi: 10.1080/19490976.2023.2235067. Gut Microbes. 2023. PMID: 37526383 Free PMC article. Review.
-
Carbohydrate Metabolism in Bacteria: Alternative Specificities in ADP-Glucose Pyrophosphorylases Open Novel Metabolic Scenarios and Biotechnological Tools.Front Microbiol. 2022 Apr 27;13:867384. doi: 10.3389/fmicb.2022.867384. eCollection 2022. Front Microbiol. 2022. PMID: 35572620 Free PMC article.
-
Structure, function, and evolution of plant ADP-glucose pyrophosphorylase.Plant Mol Biol. 2022 Mar;108(4-5):307-323. doi: 10.1007/s11103-021-01235-8. Epub 2022 Jan 10. Plant Mol Biol. 2022. PMID: 35006475 Review.
References
-
- Preiss J. 2009. Glycogen biosynthesis, p 145–158. In Schaechter M. (ed), Encyclopedia of microbiology. Academic Press (Elsevier), Cambridge, MA.
-
- Iglesias AA, Preiss J. 1992. Bacterial glycogen and plant starch biosynthesis. Biochem Educ 20:196–203. doi: 10.1016/0307-4412(92)90191-N. - DOI
-
- Ballicora MA, Erben ED, Yazaki T, Bertolo AL, Demonte AM, Schmidt JR, Aleanzi M, Bejar CM, Figueroa CM, Fusari CM, Iglesias AA, Preiss J. 2007. Identification of regions critically affecting kinetics and allosteric regulation of the Escherichia coli ADP-glucose pyrophosphorylase by modeling and pentapeptide-scanning mutagenesis. J Bacteriol 189:5325–5333. doi: 10.1128/JB.00481-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

