Brain Circuits Mediating Opposing Effects on Emotion and Pain
- PMID: 29941444
- PMCID: PMC6041794
- DOI: 10.1523/JNEUROSCI.2780-17.2018
Brain Circuits Mediating Opposing Effects on Emotion and Pain
Abstract
The amygdala is important for processing emotion, including negative emotion such as anxiety and depression induced by chronic pain. Although remarkable progress has been achieved in recent years on amygdala regulation of both negative (fear) and positive (reward) behavioral responses, our current understanding is still limited regarding how the amygdala processes and integrates these negative and positive emotion responses within the amygdala circuits. In this study with optogenetic stimulation of specific brain circuits, we investigated how amygdala circuits regulate negative and positive emotion behaviors, using pain as an emotional assay in male rats. We report here that activation of the excitatory pathway from the parabrachial nucleus (PBN) that relays peripheral pain signals to the central nucleus of amygdala (CeA) is sufficient to cause behaviors of negative emotion including anxiety, depression, and aversion in normal rats. In strong contrast, activation of the excitatory pathway from basolateral amygdala (BLA) that conveys processed corticolimbic signals to CeA dramatically opposes these behaviors of negative emotion, reducing anxiety and depression, and induces behavior of reward. Surprisingly, activating the PBN-CeA pathway to simulate pain signals does not change pain sensitivity itself, but activating the BLA-CeA pathway inhibits basal and sensitized pain. These findings demonstrate that the pain signal conveyed through the PBN-CeA pathway is sufficient to drive negative emotion and that the corticolimbic signal via the BLA-CeA pathway counteracts the negative emotion, suggesting a top-down brain mechanism for cognitive control of negative emotion under stressful environmental conditions such as pain.SIGNIFICANCE STATEMENT It remains unclear how the amygdala circuits integrate both negative and positive emotional responses and the brain circuits that link peripheral pain to negative emotion are largely unknown. Using optogenetic stimulation, this study shows that the excitatory projection from the parabrachial nucleus to the central nucleus of amygdala (CeA) is sufficient to drive behaviors of negative emotion including anxiety, depression, and aversion in rats. Conversely, activation of the excitatory projection from basolateral amygdala to CeA counteracts each of these behaviors of negative emotion. Thus, this study identifies a brain pathway that mediates pain-driven negative emotion and a brain pathway that counteracts these emotion behaviors in a top-down mechanism for brain control of negative emotion.
Keywords: amygdala; brain circuits; emotion; optogenetics; pain.
Copyright © 2018 the authors 0270-6474/18/386340-10$15.00/0.
Figures


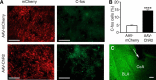
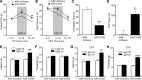

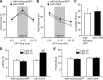
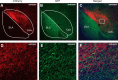
Similar articles
-
GABAergic CaMKIIα+ Amygdala Output Attenuates Pain and Modulates Emotional-Motivational Behavior via Parabrachial Inhibition.J Neurosci. 2022 Jul 6;42(27):5373-5388. doi: 10.1523/JNEUROSCI.2067-21.2022. Epub 2022 Jun 6. J Neurosci. 2022. PMID: 35667849 Free PMC article.
-
The projection from dorsal medial prefrontal cortex to basolateral amygdala promotes behaviors of negative emotion in rats.Front Neurosci. 2024 Jan 24;18:1331864. doi: 10.3389/fnins.2024.1331864. eCollection 2024. Front Neurosci. 2024. PMID: 38327845 Free PMC article.
-
Dynorphinergic Projections from the Central Amygdala to the Parabrachial Nucleus Regulate Itch.J Neurosci. 2023 Jul 19;43(29):5340-5349. doi: 10.1523/JNEUROSCI.0726-23.2023. Epub 2023 Jun 30. J Neurosci. 2023. PMID: 37399333 Free PMC article.
-
Input-specific contributions to valence processing in the amygdala.Learn Mem. 2016 Sep 15;23(10):534-43. doi: 10.1101/lm.037887.114. Print 2016 Oct. Learn Mem. 2016. PMID: 27634144 Free PMC article. Review.
-
Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors.Brain Res. 2013 May 20;1511:73-92. doi: 10.1016/j.brainres.2012.11.001. Epub 2012 Nov 8. Brain Res. 2013. PMID: 23142759 Free PMC article. Review.
Cited by
-
Effects of an Intrathecal Drug Delivery System Connected to a Subcutaneous Port on Pain, Mood and Quality of Life in End Stage Cancer Patients: An Observational Study.Cancer Control. 2022 Jan-Dec;29:10732748221133752. doi: 10.1177/10732748221133752. Cancer Control. 2022. PMID: 36281899 Free PMC article.
-
Psychotherapy in pain management: New viewpoints and treatment targets based on a brain theory.AIMS Neurosci. 2020 Jul 14;7(3):194-207. doi: 10.3934/Neuroscience.2020013. eCollection 2020. AIMS Neurosci. 2020. PMID: 32995484 Free PMC article.
-
PBN-PVT projections modulate negative affective states in mice.Elife. 2022 Feb 15;11:e68372. doi: 10.7554/eLife.68372. Elife. 2022. PMID: 35167440 Free PMC article.
-
Parabrachial Nucleus Activity in Nociception and Pain in Awake Mice.J Neurosci. 2023 Aug 2;43(31):5656-5667. doi: 10.1523/JNEUROSCI.0587-23.2023. Epub 2023 Jul 14. J Neurosci. 2023. PMID: 37451980 Free PMC article.
-
Neural circuits regulating visceral pain.Commun Biol. 2024 Apr 13;7(1):457. doi: 10.1038/s42003-024-06148-y. Commun Biol. 2024. PMID: 38615103 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
