Axonal Ensheathment in the Nervous System of Lamprey: Implications for the Evolution of Myelinating Glia
- PMID: 29941446
- PMCID: PMC6705951
- DOI: 10.1523/JNEUROSCI.1034-18.2018
Axonal Ensheathment in the Nervous System of Lamprey: Implications for the Evolution of Myelinating Glia
Abstract
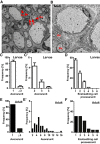
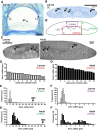
In the nervous system, myelination of axons enables rapid impulse conduction and is a specialized function of glial cells. Myelinating glia are the last cell type to emerge in the evolution of vertebrate nervous systems, presumably in ancient jawed vertebrates (gnathostomata) because jawless vertebrates (agnathans) lack myelin. We have hypothesized that, in these unmyelinated species, evolutionary progenitors of myelinating cells must have existed that should still be present in contemporary agnathan species. Here, we used advanced electron microscopic techniques to reveal axon-glia interactions in the sea lamprey Petromyzon marinus By quantitative assessment of the spinal cord and the peripheral lateral line nerve, we observed a marked maturation-dependent growth of axonal calibers. In peripheral nerves, all axons are ensheathed by glial cells either in bundles or, when larger than the threshold caliber of 3 μm, individually. The ensheathing glia are covered by a basal lamina and express SoxE-transcription factors, features of mammalian Remak-type Schwann cells. In larval lamprey, the ensheathment of peripheral axons leaves gaps that are closed in adults. CNS axons are also covered to a considerable extent by glial processes, which contain a high density of intermediate filaments, glycogen particles, large lipid droplets, and desmosomes, similar to mammalian astrocytes. Indeed, by in situ hybridization, these glial cells express the astrocyte marker Aldh1l1 Specimens were of unknown sex. Our observations imply that radial sorting, ensheathment, and presumably also metabolic support of axons are ancient functions of glial cells that predate the evolutionary emergence of myelin in jawed vertebrates.SIGNIFICANCE STATEMENT We used current electron microscopy techniques to examine axon-glia units in a nonmyelinated vertebrate species, the sea lamprey. In the PNS, lamprey axons are fully ensheathed either individually or in bundles by cells ortholog to Schwann cells. In the CNS, axons associate with astrocyte orthologs, which contain glycogen and lipid droplets. We suggest that ensheathment, radial sorting, and metabolic support of axons by glial cells predate the evolutionary emergence of myelin in ancient jawed vertebrates.
Keywords: Schwann cell; axon–glia interaction; electron microscopy; myelin; oligodendrocyte; radial sorting.
Copyright © 2018 the authors 0270-6474/18/386586-11$15.00/0.
Figures


Similar articles
-
Ensheathment and Myelination of Axons: Evolution of Glial Functions.Annu Rev Neurosci. 2021 Jul 8;44:197-219. doi: 10.1146/annurev-neuro-100120-122621. Epub 2021 Mar 15. Annu Rev Neurosci. 2021. PMID: 33722070
-
Gliogenesis in lampreys shares gene regulatory interactions with oligodendrocyte development in jawed vertebrates.Dev Biol. 2018 Sep 1;441(1):176-190. doi: 10.1016/j.ydbio.2018.07.002. Epub 2018 Jul 4. Dev Biol. 2018. PMID: 29981309
-
Schwann cells in the regenerating fish optic nerve: evidence that CNS axons, not the glia, determine when myelin formation begins.J Neurocytol. 2000 Apr;29(4):285-300. doi: 10.1023/a:1026575805331. J Neurocytol. 2000. PMID: 11276180
-
Analysis of myelinated axon formation in zebrafish.Methods Cell Biol. 2017;138:383-414. doi: 10.1016/bs.mcb.2016.08.001. Epub 2016 Sep 29. Methods Cell Biol. 2017. PMID: 28129853 Free PMC article. Review.
-
The acquisition of myelin: a success story.Novartis Found Symp. 2006;276:15-21; discussion 21-5, 54-7, 275-81. doi: 10.1002/9780470032244.ch3. Novartis Found Symp. 2006. PMID: 16805421 Review.
Cited by
-
Mice carrying an analogous heterozygous dynamin 2 K562E mutation that causes neuropathy in humans develop predominant characteristics of a primary myopathy.Hum Mol Genet. 2020 May 28;29(8):1253-1273. doi: 10.1093/hmg/ddaa034. Hum Mol Genet. 2020. PMID: 32129442 Free PMC article.
-
Gene Profiling in the Adipose Fin of Salmonid Fishes Supports its Function as a Flow Sensor.Genes (Basel). 2019 Dec 23;11(1):21. doi: 10.3390/genes11010021. Genes (Basel). 2019. PMID: 31878086 Free PMC article.
-
A cell type atlas of the lamprey brain.Nat Ecol Evol. 2023 Oct;7(10):1591-1592. doi: 10.1038/s41559-023-02195-6. Nat Ecol Evol. 2023. PMID: 37710040 No abstract available.
-
Developmental axon diameter growth of central nervous system axons does not depend on ensheathment or myelination by oligodendrocytes.bioRxiv [Preprint]. 2025 Jan 10:2025.01.10.632348. doi: 10.1101/2025.01.10.632348. bioRxiv. 2025. PMID: 39829751 Free PMC article. Preprint.
-
Metabolic Interaction Between Schwann Cells and Axons Under Physiological and Disease Conditions.Front Cell Neurosci. 2020 May 29;14:148. doi: 10.3389/fncel.2020.00148. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32547370 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
