Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05-001
- PMID: 29941458
- PMCID: PMC6020806
- DOI: 10.1182/bloodadvances.2018016584
Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05-001
Abstract
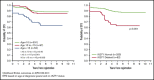
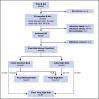
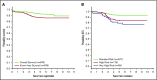
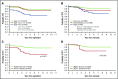
Dana-Farber Cancer Institute (DFCI) ALL Consortium Protocol 05-001 tested a new risk stratification system in children and adolescents with newly diagnosed acute lymphoblastic leukemia (ALL). At study entry, B-ALL patients were classified as standard risk (SR) or high risk (HR) based on age, white blood cell (WBC) count, and central nervous system status. After achieving complete remission (CR), patients with high end-induction minimal residual disease (MRD) (≥10-3 by polymerase chain reaction analysis of patient-specific antigen receptor rearrangements) and/or adverse cytogenetics (KMT2A rearrangement or hypodiploidy) were reclassified as very high risk (VHR) and received intensified therapy. IKZF1 deletion status was retrospectively evaluated by multiplex ligation-dependent probe amplification. Between 2005 and 2011, 678 Philadelphia chromosome-negative B-ALL patients aged 1 to 18 years enrolled; 651 achieved CR and 648 received a final risk group. Among all 678 patients, 5-year event-free survival (EFS) was 87% (95% confidence interval [CI], 84-89) and overall survival 93% (95% CI, 90-94). Five-year disease-free survival of SR patients (N = 407) was 94% (95% CI, 91-96), HR (N = 176) was 84% (95% CI, 77-88), and VHR (N = 65) was 79% (95% CI, 67-87). IKZF1 deletion was present in 62 of 385 (16%) assessed patients and was associated with inferior 5-year EFS (63%; 95% CI, 49%-74% vs 88%; 95% CI, 84%-91%; P < .001), and higher 5-year cumulative incidence of relapse, including among those with low MRD (24% vs 8%, P = .001). In multivariable analysis, age ≥15 years, WBC ≥50 × 109/L, IKZF1 deletion, and MRD ≥10-4 was each associated with inferior outcome. In conclusion, risk-stratified therapy on DFCI 05-001 resulted in favorable outcomes for B-ALL patients, including those with VHR features. IKZF1 deletion was an independent predictor of inferior outcome. This trial was registered at www.clinicaltrials.gov as #NCT00400946.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.L.A. has served on advisory boards for Sigma Tau Pharmaceuticals and advisory boards and speakers bureau for Jazz Pharmaceuticals. L.B.S. has served on advisory boards for Sigma Tau Pharmaceuticals, Jazz Pharmaceuticals, and Baxalta. The remaining authors declare no competing financial interests.
Figures
References
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18-24. - PubMed
-
- Moorman AV, Ensor HM, Richards SM, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010;11(5):429-438. - PubMed
-
- Pui CH, Chessells JM, Camitta B, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17(4):700-706. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

