Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth
- PMID: 29941600
- PMCID: PMC6048537
- DOI: 10.1073/pnas.1720113115
Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth
Abstract
The tumor microenvironment restrains conventional T cell (Tconv) activation while facilitating the expansion of Tregs. Here we showed that Tregs' advantage in the tumor milieu relies on supplemental energetic routes involving lipid metabolism. In murine models, tumor-infiltrating Tregs displayed intracellular lipid accumulation, which was attributable to an increased rate of fatty acid (FA) synthesis. Since the relative advantage in glucose uptake may fuel FA synthesis in intratumoral Tregs, we demonstrated that both glycolytic and oxidative metabolism contribute to Tregs' expansion. We corroborated our data in human tumors showing that Tregs displayed a gene signature oriented toward glycolysis and lipid synthesis. Our data support a model in which signals from the tumor microenvironment induce a circuitry of glycolysis, FA synthesis, and oxidation that confers a preferential proliferative advantage to Tregs, whose targeting might represent a strategy for cancer treatment.
Keywords: Treg; fatty acid synthesis; glycolysis; ox40; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
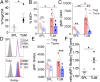

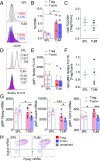

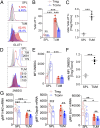
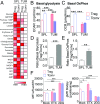

References
-
- Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol. 2015;33:101–111. - PubMed
-
- Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. - PubMed
-
- Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

