Pallidal Stimulation Modulates Pedunculopontine Nuclei in Parkinson's Disease
- PMID: 29941788
- PMCID: PMC6071240
- DOI: 10.3390/brainsci8070117
Pallidal Stimulation Modulates Pedunculopontine Nuclei in Parkinson's Disease
Abstract
Background: In advanced Parkinson’s disease, the pedunculopontine nucleus region is thought to be abnormally inhibited by gamma-aminobutyric acid (GABA) ergic inputs from the over-active globus pallidus internus. Recent attempts to boost pedunculopontine nucleus function through deep brain stimulation are promising, but suffer from the incomplete understanding of the physiology of the pedunculopontine nucleus region.
Methods: Local field potentials of the pedunculopontine nucleus region and the globus pallidus internus were recorded and quantitatively analyzed in a patient with Parkinson’s disease. In particular, we compared the local field potentials from the pedunculopontine nucleus region at rest and during deep brain stimulation of the globus pallidus internus.
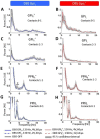
Results: At rest, the spectrum of local field potentials in the globus pallidus internus was mainly characterized by delta-theta and beta frequency activity whereas the spectrum of the pedunculopontine nucleus region was dominated by activity only in the delta and theta band. High-frequency deep brain stimulation of the globus pallidus internus led to increased theta activity in the pedunculopontine nucleus region and enabled information exchange between the left and right pedunculopontine nuclei. Therefore, Conclusions: When applying deep brain stimulation in the globus pallidus internus, its modulatory effect on pedunculopontine nucleus physiology should be taken into account.
Keywords: Parkinson’s disease; deep brain stimulation; globus pallidus internus; local field potentials; pedunculopontine nucleus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Effects of pedunculopontine area and pallidal DBS on gait ignition in Parkinson's disease.Brain Stimul. 2013 Nov;6(6):856-9. doi: 10.1016/j.brs.2013.05.005. Epub 2013 Jun 10. Brain Stimul. 2013. PMID: 23791131
-
Parkinson's disease outcomes after intraoperative CT-guided "asleep" deep brain stimulation in the globus pallidus internus.J Neurosurg. 2016 Apr;124(4):902-7. doi: 10.3171/2015.4.JNS1550. Epub 2015 Oct 9. J Neurosurg. 2016. PMID: 26452116
-
Pallidal Deep-Brain Stimulation Disrupts Pallidal Beta Oscillations and Coherence with Primary Motor Cortex in Parkinson's Disease.J Neurosci. 2018 May 9;38(19):4556-4568. doi: 10.1523/JNEUROSCI.0431-18.2018. Epub 2018 Apr 16. J Neurosci. 2018. PMID: 29661966 Free PMC article.
-
Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation for the Treatment of Patients With Parkinson's Disease: Executive Summary.Neurosurgery. 2018 Jun 1;82(6):753-756. doi: 10.1093/neuros/nyy037. Neurosurgery. 2018. PMID: 29538685 Free PMC article.
-
Criteria for deep-brain stimulation in Parkinson's disease: review and analysis.Expert Rev Neurother. 2006 Nov;6(11):1695-705. doi: 10.1586/14737175.6.11.1695. Expert Rev Neurother. 2006. PMID: 17144783 Review.
Cited by
-
Anatomical Characterization of the Human Structural Connectivity between the Pedunculopontine Nucleus and Globus Pallidus via Multi-Shell Multi-Tissue Tractography.Medicina (Kaunas). 2020 Sep 7;56(9):452. doi: 10.3390/medicina56090452. Medicina (Kaunas). 2020. PMID: 32906651 Free PMC article.
References
-
- Stefani A., Lozano A.M., Peppe A., Stanzione P., Galati S., Tropepi D., Pierantozzi M., Brusa L., Scarnati E., Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain J. Neurol. 2007;130:1596–1607. doi: 10.1093/brain/awl346. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

