2-[2-(4-(trifluoromethyl)phenylamino)thiazol-4-yl]acetic acid (Activator-3) is a potent activator of AMPK
- PMID: 29942003
- PMCID: PMC6018554
- DOI: 10.1038/s41598-018-27974-1
2-[2-(4-(trifluoromethyl)phenylamino)thiazol-4-yl]acetic acid (Activator-3) is a potent activator of AMPK
Abstract
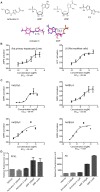
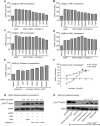
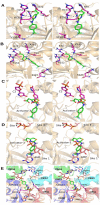
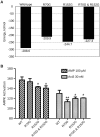
AMPK is considered as a potential high value target for metabolic disorders. Here, we present the molecular modeling, in vitro and in vivo characterization of Activator-3, 2-[2-(4-(trifluoromethyl)phenylamino)thiazol-4-yl]acetic acid, an AMP mimetic and a potent pan-AMPK activator. Activator-3 and AMP likely share common activation mode for AMPK activation. Activator-3 enhanced AMPK phosphorylation by upstream kinase LKB1 and protected AMPK complex against dephosphorylation by PP2C. Molecular modeling analyses followed by in vitro mutant AMPK enzyme assays demonstrate that Activator-3 interacts with R70 and R152 of the CBS1 domain on AMPK γ subunit near AMP binding site. Activator-3 and C2, a recently described AMPK mimetic, bind differently in the γ subunit of AMPK. Activator-3 unlike C2 does not show cooperativity of AMPK activity in the presence of physiological concentration of ATP (2 mM). Activator-3 displays good pharmacokinetic profile in rat blood plasma with minimal brain penetration property. Oral treatment of High Sucrose Diet (HSD) fed diabetic rats with 10 mg/kg dose of Activator-3 once in a day for 30 days significantly enhanced glucose utilization, improved lipid profiles and reduced body weight, demonstrating that Activator-3 is a potent AMPK activator that can alleviate the negative metabolic impact of high sucrose diet in rat model.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Henry RR, et al. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study. Lancet Lond. Engl. 2009;374:126–135. doi: 10.1016/S0140-6736(09)60870-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

