Mutually exclusive acetylation and ubiquitylation of the splicing factor SRSF5 control tumor growth
- PMID: 29942010
- PMCID: PMC6018636
- DOI: 10.1038/s41467-018-04815-3
Mutually exclusive acetylation and ubiquitylation of the splicing factor SRSF5 control tumor growth
Abstract
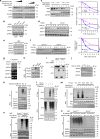
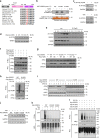
Most tumor cells take up more glucose than normal cells. Splicing dysregulation is one of the molecular hallmarks of cancer. However, the role of splicing factor in glucose metabolism and tumor development remains poorly defined. Here, we show that upon glucose intake, the splicing factor SRSF5 is specifically induced through Tip60-mediated acetylation on K125, which antagonizes Smurf1-mediated ubiquitylation. SRSF5 promotes the alternative splicing of CCAR1 to produce CCAR1S proteins, which promote tumor growth by enhancing glucose consumption and acetyl-CoA production. Conversely, upon glucose starvation, SRSF5 is deacetylated by HDAC1, and ubiquitylated by Smurf1 on the same lysine, resulting in proteasomal degradation of SRSF5. The CCAR1L proteins accumulate to promote apoptosis. Importantly, SRSF5 is hyperacetylated and upregulated in human lung cancers, which correlates with increased CCAR1S expression and tumor progression. Thus, SRSF5 responds to high glucose to promote cancer development, and SRSF5-CCAR1 axis may be valuable targets for cancer therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
SRSF5 functions as a novel oncogenic splicing factor and is upregulated by oncogene SRSF3 in oral squamous cell carcinoma.Biochim Biophys Acta Mol Cell Res. 2018 Sep;1865(9):1161-1172. doi: 10.1016/j.bbamcr.2018.05.017. Epub 2018 May 30. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29857020
-
Antitumor activity of SR splicing-factor 5 knockdown by downregulating pyruvate kinase M2 in non-small cell lung cancer cells.J Cell Biochem. 2019 Oct;120(10):17303-17311. doi: 10.1002/jcb.28992. Epub 2019 May 20. J Cell Biochem. 2019. PMID: 31106485
-
CLK1/SRSF5 pathway induces aberrant exon skipping of METTL14 and Cyclin L2 and promotes growth and metastasis of pancreatic cancer.J Hematol Oncol. 2021 Apr 13;14(1):60. doi: 10.1186/s13045-021-01072-8. J Hematol Oncol. 2021. PMID: 33849617 Free PMC article.
-
SRSF5: a novel marker for small-cell lung cancer and pleural metastatic cancer.Lung Cancer. 2016 Sep;99:57-65. doi: 10.1016/j.lungcan.2016.05.018. Epub 2016 May 26. Lung Cancer. 2016. PMID: 27565915
-
CCAR1 and CCAR2 as gene chameleons with antagonistic duality: Preclinical, human translational, and mechanistic basis.Cancer Sci. 2020 Oct;111(10):3416-3425. doi: 10.1111/cas.14579. Epub 2020 Aug 9. Cancer Sci. 2020. PMID: 33403784 Free PMC article. Review.
Cited by
-
Proteomics separates adult-type diffuse high-grade gliomas in metabolic subgroups independent of 1p/19q codeletion and across IDH mutational status.Cell Rep Med. 2023 Jan 17;4(1):100877. doi: 10.1016/j.xcrm.2022.100877. Epub 2022 Dec 29. Cell Rep Med. 2023. PMID: 36584682 Free PMC article.
-
Targeting fibrinogen-like protein 1 enhances immunotherapy in hepatocellular carcinoma.J Clin Invest. 2023 May 1;133(9):e164528. doi: 10.1172/JCI164528. J Clin Invest. 2023. PMID: 37115693 Free PMC article.
-
Targeting SRSF10 might inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy in hepatocellular carcinoma.Cancer Commun (Lond). 2024 Nov;44(11):1231-1260. doi: 10.1002/cac2.12607. Epub 2024 Sep 2. Cancer Commun (Lond). 2024. PMID: 39223929 Free PMC article.
-
The crosstalk among the physical tumor microenvironment and the effects of glucose deprivation on tumors in the past decade.Front Cell Dev Biol. 2023 Nov 1;11:1275543. doi: 10.3389/fcell.2023.1275543. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020920 Free PMC article. Review.
-
Inhibiting Arginine Methylation as a Tool to Investigate Cross-Talk with Methylation and Acetylation Post-Translational Modifications in a Glioblastoma Cell Line.Proteomes. 2018 Oct 20;6(4):44. doi: 10.3390/proteomes6040044. Proteomes. 2018. PMID: 30347783 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

