α7 Nicotinic acetylcholine receptor-mediated anti-inflammatory effect in a chronic migraine rat model via the attenuation of glial cell activation
- PMID: 29942148
- PMCID: PMC6007207
- DOI: 10.2147/JPR.S159146
α7 Nicotinic acetylcholine receptor-mediated anti-inflammatory effect in a chronic migraine rat model via the attenuation of glial cell activation
Abstract
Background: Evidence suggests that the activation of α7 nicotinic acetylcholine receptor (α7nAChR) can greatly decrease the neuroinflammation response. Neuroinflammation plays a pivotal role in the pathogenesis of chronic migraine (CM). Clinical observations also show that nicotine gum induces analgesic effects in migraine patients. However, whether α7nAChR is involved in CM is unclear.
Objective: To investigate the role of α7nAChR in CM and provide a new therapeutic target for CM.
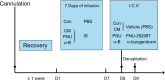
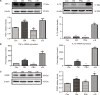
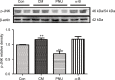
Materials and methods: Thirty-six male Sprague-Dawley rats were distributed randomly into control, CM, PNU-282987, and α-bungarotoxin groups (n=9 rats in each group). The CM model was established by the recurrent daily administration of inflammatory soup on the dura over the course of 1 week. The hind paw threshold and facial allodynia were assessed by the von Frey test. The expression levels of α7nAChR, tumor necrosis factor-alpha, and interleukin-1 beta were analyzed by Western blot and real-time fluorescence quantitative polymerase chain reaction. The location of α7nAChR in the hippocampus was quantified by immunofluorescence, as well as the microglial and astrocyte alterations. Changes in the calcitonin gene-related peptide and the phosphorylated JNK protein among different groups were measured by Western blot.
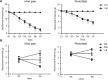
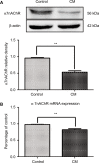
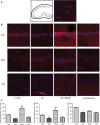
Results: We found that the expression of α7nAChR was reduced after repeated inflammatory soup administration. The increased expression of tumor necrosis factor-alpha, interleukin-1 beta, and calcitonin gene-related peptide in CM group were significantly decreased by PNU-282987 and aggravated by α-bungarotoxin. Moreover, PNU-282987 decreased the numbers of astrocytes and microglia compared with the numbers in the CM group in both hippocampal CA1 and CA3 regions. In contrast, α-bungarotoxin activated the astrocytes and microglia, but the differences with respect to the CM group were not significant. Activated c-Jun N-terminal kinase signaling was observed in CM rats and was also blocked by PNU-282987.
Conclusion: The activation of α7nAChR increased the mechanical threshold and alleviated pain in the CM rat model. α7nAChR activation also decreased the upregulation of astrocytes and microglia through the p-c-Jun N-terminal kinase-mitogen-activated protein kinase signaling pathway.
Keywords: analgesia; chronic migraine; glial activation; neuroinflammation; nociception; α7nAChR.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
α7nAChR-mediated astrocytic activation: A novel mechanism of Xiongzhi Dilong decoction in ameliorating chronic migraine.J Ethnopharmacol. 2024 Nov 15;334:118509. doi: 10.1016/j.jep.2024.118509. Epub 2024 Jul 4. J Ethnopharmacol. 2024. PMID: 38971346
-
[The effects of vagus nerve stimulation on hippocampal neuro-inflammatory and α7nAChR expression in rats with intractable epilepsy].Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022 Jul;38(4):373-378. doi: 10.12047/j.cjap.6274.2022.070. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022. PMID: 36414564 Chinese.
-
Activation of α7 Nicotinic Acetylcholine Receptor Protects Against 1-Methyl-4-Phenylpyridinium-Induced Astroglial Apoptosis.Front Cell Neurosci. 2019 Nov 12;13:507. doi: 10.3389/fncel.2019.00507. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31780901 Free PMC article.
-
New Insight into Neuropathic Pain: The Relationship between α7nAChR, Ferroptosis, and Neuroinflammation.Int J Mol Sci. 2024 Jun 18;25(12):6716. doi: 10.3390/ijms25126716. Int J Mol Sci. 2024. PMID: 38928421 Free PMC article. Review.
-
Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: a narrative review.Ann Transl Med. 2021 Mar;9(6):509. doi: 10.21037/atm-21-273. Ann Transl Med. 2021. PMID: 33850906 Free PMC article. Review.
Cited by
-
Mechanisms of comorbidity between Alzheimer's disease and pain.Alzheimers Dement. 2025 Feb;21(2):e14605. doi: 10.1002/alz.14605. Alzheimers Dement. 2025. PMID: 39998175 Free PMC article. Review.
-
Alpha-7 Nicotinic Receptor Agonist Protects Mice Against Pulmonary Emphysema Induced by Elastase.Inflammation. 2024 Jun;47(3):958-974. doi: 10.1007/s10753-023-01953-9. Epub 2024 Jan 16. Inflammation. 2024. PMID: 38227123
-
Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation.World J Stem Cells. 2021 Aug 26;13(8):1072-1083. doi: 10.4252/wjsc.v13.i8.1072. World J Stem Cells. 2021. PMID: 34567426 Free PMC article. Review.
-
Cholinergic Modulation of Glial Function During Aging and Chronic Neuroinflammation.Front Cell Neurosci. 2020 Oct 15;14:577912. doi: 10.3389/fncel.2020.577912. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192323 Free PMC article. Review.
-
Could Experimental Inflammation Provide Better Understanding of Migraines?Cells. 2022 Aug 6;11(15):2444. doi: 10.3390/cells11152444. Cells. 2022. PMID: 35954288 Free PMC article. Review.
References
-
- Bigal ME, Rapoport AM, Lipton RB, Tepper SJ, Sheftell FD. Assessment of Migraine Disability Using the Migraine Disability Assessment (MIDAS) questionnaire: a comparison of chronic migraine with episodic migraine. Headache. 2003;43(4):336–342. - PubMed
-
- Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47(7):1050–1055. - PubMed
-
- Domínguez C, Vieites-Prado A, Pérez-Mato M, et al. Role of adipocytokines in the pathophysiology of migraine: a cross-sectional study. Cephalalgia. 2017;35(5):904–911. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

