Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: a systematic review
- PMID: 29942170
- PMCID: PMC6005317
- DOI: 10.2147/AHMT.S163121
Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: a systematic review
Abstract
Purpose: Although cancer clinical trials (CT) offer opportunities for novel treatments that may lead to improved outcomes, adolescents and young adults (AYA) are less likely to participate in these trials as compared to younger children and older adults. We aimed to identify the perceptions and attitudes toward CT in AYA that influence trial participation.
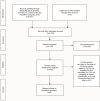
Materials and methods: A systematic review of cancer literature was conducted that assessed perceptions and attitudes toward CT enrollment limited to AYA patients (defined as age 15-39). We estimated the frequency of identified themes by pooling identified studies.
Results: In total, six original research articles were identified that specifically addressed perceptions or attitudes that influenced CT participation in AYA patients. Three studies were conducted at pediatric centers - one at an AYA unit, one at an adult cancer hospital, and one was registry based. Major themes identified for CT acceptability included: hope for positive clinical affect, altruism, and having autonomy. Potential deterrents included: prolonged hospitalization, worry of side effects, and discomfort with experimentation.
Conclusion: Limited information is available with regard to the perceptions and attitudes toward CT acceptability among AYA patients, especially those treated at adult cancer centers, which prevents generalization of data and themes. Future research assessing strategies for understanding and supporting CT decision-making processes among AYA represents a key focus for future funding to improve CT enrollment.
Keywords: adolescent and young adult; barriers; cancer; clinical trials; psychosocial.
Conflict of interest statement
Disclosure Abha A Gupta and Jeremy Lewin share senior authorship. The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: Identifying opportunities for intervention.Cancer. 2020 Mar 1;126(5):949-957. doi: 10.1002/cncr.32675. Epub 2019 Dec 23. Cancer. 2020. PMID: 31869454 Free PMC article.
-
Evaluation of the Pediatric Research Participation Questionnaire for Measuring Attitudes Toward Cancer Clinical Trials Among Adolescents and Young Adults.J Adolesc Young Adult Oncol. 2019 Aug;8(4):423-433. doi: 10.1089/jayao.2018.0144. Epub 2019 Apr 26. J Adolesc Young Adult Oncol. 2019. PMID: 31025898 Free PMC article.
-
Perceptions of and decision making about clinical trials in adolescent and young adults with Cancer: a qualitative analysis.BMC Cancer. 2018 Jun 4;18(1):629. doi: 10.1186/s12885-018-4515-2. BMC Cancer. 2018. PMID: 29866065 Free PMC article.
-
A qualitative study of barriers and facilitators to adolescents and young adults' participation in cancer clinical trials: Oncologist and patient perspectives.Pediatr Blood Cancer. 2022 Apr;69(4):e29479. doi: 10.1002/pbc.29479. Epub 2021 Dec 16. Pediatr Blood Cancer. 2022. PMID: 34913583
-
Quality of life in adolescent and young adult cancer patients: a systematic review of the literature.Patient Relat Outcome Meas. 2015 Feb 17;6:19-51. doi: 10.2147/PROM.S51658. eCollection 2015. Patient Relat Outcome Meas. 2015. PMID: 25733941 Free PMC article. Review.
Cited by
-
Closing the gap: proposing a socio-ecological framework to make cancer clinical trials more accessible, equitable, and acceptable to adolescents and young adults.Oncologist. 2024 Nov 4;29(11):918-921. doi: 10.1093/oncolo/oyae257. Oncologist. 2024. PMID: 39331472 Free PMC article.
-
Views of Adolescents and Young Adults with Cancer and Their Oncologists Toward Patients' Participation in Genomic Research.J Adolesc Young Adult Oncol. 2023 Oct;12(5):773-781. doi: 10.1089/jayao.2022.0066. Epub 2023 Jan 2. J Adolesc Young Adult Oncol. 2023. PMID: 36595372 Free PMC article.
-
Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: Identifying opportunities for intervention.Cancer. 2020 Mar 1;126(5):949-957. doi: 10.1002/cncr.32675. Epub 2019 Dec 23. Cancer. 2020. PMID: 31869454 Free PMC article.
-
Motivations and experiences of patients with respiratory disease and their caregivers in a multinational trial of mirtazapine for severe breathlessness: a qualitative study (BETTER-B).BMC Pulm Med. 2025 Aug 14;25(1):390. doi: 10.1186/s12890-025-03862-z. BMC Pulm Med. 2025. PMID: 40813667 Free PMC article.
-
Shared barriers and facilitators to enrollment of adolescents and young adults on cancer clinical trials.Sci Rep. 2022 Mar 9;12(1):3875. doi: 10.1038/s41598-022-07703-5. Sci Rep. 2022. PMID: 35264642 Free PMC article.
References
-
- Fern LA, Whelan JS. Recruitment of adolescents and young adults to cancer clinical trials—international comparisons, barriers, and implications. Semin Oncol. 2010;37(2):e1–e8. - PubMed
-
- Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J Pediatr Hematol Oncol. 2009;31(12):927–929. - PubMed
-
- Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363(9405):263–270. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

