DN2 Thymocytes Activate a Specific Robust DNA Damage Response to Ionizing Radiation-Induced DNA Double-Strand Breaks
- PMID: 29942310
- PMCID: PMC6004388
- DOI: 10.3389/fimmu.2018.01312
DN2 Thymocytes Activate a Specific Robust DNA Damage Response to Ionizing Radiation-Induced DNA Double-Strand Breaks
Abstract
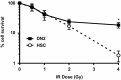
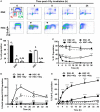
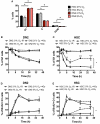
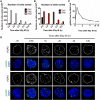
For successful bone marrow transplantation (BMT), a preconditioning regime involving chemo and radiotherapy is used that results in DNA damage to both hematopoietic and stromal elements. Following radiation exposure, it is well recognized that a single wave of host-derived thymocytes reconstitutes the irradiated thymus, with donor-derived thymocytes appearing about 7 days post BMT. Our previous studies have demonstrated that, in the presence of donor hematopoietic cells lacking T lineage potential, these host-derived thymocytes are able to generate a polyclonal cohort of functionally mature peripheral T cells numerically comprising ~25% of the peripheral T cell pool of euthymic mice. Importantly, we demonstrated that radioresistant CD44+ CD25+ CD117+ DN2 progenitors were responsible for this thymic auto-reconstitution. Until recently, the mechanisms underlying the radioresistance of DN2 progenitors were unknown. Herein, we have used the in vitro "Plastic Thymus" culture system to perform a detailed investigation of the mechanisms responsible for the high radioresistance of DN2 cells compared with radiosensitive hematopoietic stem cells. Our results indicate that several aspects of DN2 biology, such as (i) rapid DNA damage response (DDR) activation in response to ionizing radiation-induced DNA damage, (ii) efficient repair of DNA double-strand breaks, and (iii) induction of a protective G1/S checkpoint contribute to promoting DN2 cell survival post-irradiation. We have previously shown that hypoxia increases the radioresistance of bone marrow stromal cells in vitro, at least in part by enhancing their DNA double-strand break (DNA DSB) repair capacity. Since the thymus is also a hypoxic environment, we investigated the potential effects of hypoxia on the DDR of DN2 thymocytes. Finally, we demonstrate for the first time that de novo DN2 thymocytes are able to rapidly repair DNA DSBs following thymic irradiation in vivo.
Keywords: DN2 pro-T cells; DNA damage response; bone marrow transplantation; hypoxia; ionizing radiation; thymic auto-reconstitution.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

