Ectonucleotidase-Mediated Suppression of Lupus Autoimmunity and Vascular Dysfunction
- PMID: 29942314
- PMCID: PMC6004379
- DOI: 10.3389/fimmu.2018.01322
Ectonucleotidase-Mediated Suppression of Lupus Autoimmunity and Vascular Dysfunction
Abstract
Objectives: CD39 and CD73 are surface enzymes that jut into the extracellular space where they mediate the step-wise phosphohydrolysis of the autocrine and paracrine danger signals ATP and ADP into anti-inflammatory adenosine. Given the role of vascular and immune cells' "purinergic halo" in maintaining homeostasis, we hypothesized that the ectonucleotidases CD39 and CD73 might play a protective role in lupus.
Methods: Lupus was modeled by intraperitoneal administration of pristane to three groups of mice: wild-type (WT), CD39-/-, and CD73-/-. After 36 weeks, autoantibodies, endothelial function, kidney disease, splenocyte activation/polarization, and neutrophil activation were characterized.
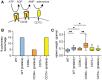
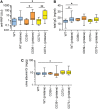
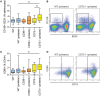
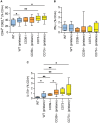
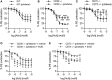
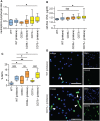
Results: As compared with WT mice, CD39-/- mice developed exaggerated splenomegaly in response to pristane, while both groups of ectonucleotidase-deficient mice demonstrated heightened anti-ribonucleoprotein production. The administration of pristane to WT mice triggered only subtle dysfunction of the arterial endothelium; however, both CD39-/- and CD73-/- mice demonstrated striking endothelial dysfunction following induction of lupus, which could be reversed by superoxide dismutase. Activated B cells and plasma cells were expanded in CD73-/- mice, while deficiency of either ectonucleotidase led to expansion of TH17 cells. CD39-/- and CD73-/- mice demonstrated exaggerated neutrophil extracellular trap release, while CD73-/- mice additionally had higher levels of plasma cell-free DNA.
Conclusion: These data are the first to link ectonucleotidases with lupus autoimmunity and vascular disease. New therapeutic strategies may harness purinergic nucleotide dissipation or signaling to limit the damage inflicted upon organs and blood vessels by lupus.
Keywords: CD39; CD73; TH17 cells; ectonucleotidases; endothelial dysfunction; neutrophil extracellular traps; systemic lupus erythematosus.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

