Metformin in the management of diabetes during pregnancy and lactation
- PMID: 29942340
- PMCID: PMC6012930
- DOI: 10.7573/dic.212523
Metformin in the management of diabetes during pregnancy and lactation
Abstract
This review explores the current place of metformin in the management of gestational diabetes (GDM) and type 2 diabetes during pregnancy and lactation. The rationale and basic pharmacology of metformin usage in pregnancy is discussed along with the evidence from observational and randomized controlled trials in women with GDM or overt diabetes. There seems to be adequate evidence of efficacy and short-term safety of metformin in relation to maternal and neonatal outcomes in GDM, with possible benefits related to lower maternal weight gain and lower risk of neonatal hypoglycemia and macrosomia. Additionally, metformin offers the advantages of oral administration, convenience, less cost and greater acceptability. Metformin may, therefore, be considered in milder forms of GDM where glycemic goals are not attained by lifestyle modification. However, failure rate is likely to be higher in those with an earlier diagnosis of GDM, higher blood glucose, higher body mass index (BMI) or previous history of GDM, and insulin remains the cornerstone of pharmacological treatment in such cases. The use of metformin in type 2 diabetes has been assessed in observational and small randomized trials. Metformin monotherapy in women with overt diabetes is highly unlikely to achieve glycemic targets. Hence, the use should be restricted as adjunct to insulin and may be considered in women with high insulin dose requirements or rapid weight gain. There is clearly a need for more clinical trials to assess the effect of combined insulin plus metformin therapy in pregnancy with type 2 diabetes. Additionally, there is a paucity of data on long-term effects in offspring exposed to metformin in utero. It is imperative to further explore its impact on offspring as metformin has significant transplacental transfer and has the potential to impact the programming of the epigenome. Therefore, caution must be exercised when prescribing metformin in pregnant women. More research is clearly needed before metformin can be considered as standard of care in the management of diabetes during pregnancy.
Keywords: diabetes; epigenetic programming; gestational diabetes; metformin; oral antidiabetic agents; pregnancy; type 2 diabetes.
Conflict of interest statement
Disclosure and potential conflicts of interest: The authors declare no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/05/dic.212523-COI.pdf
Figures
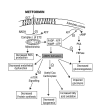

References
-
- Falavigna M, Schmidt MI, Trujillo J, Alves LF, Wendland ER, Torloni MR, Colagiuri S, Duncan BB. Effectiveness of gestational diabetes treatment: a systematic review with quality of evidence assessment. Diabetes Res Clin Pract. 2012 Dec;98(3):396–405. doi: 10.1016/j.diabres.2012.09.002. - DOI - PubMed
-
- Coetzee EJ, Jackson WP. Oral hypoglycemic in the first trimester and fetal outcome. S Afr Med J. 1984 Apr 21;65(16):635–7. - PubMed
-
- Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabetes during pregnancy. Diabetes Res Clin Pract. 1985–1986 Feb;1(5):281–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

