Variation in FPOP Measurements Is Primarily Caused by Poor Peptide Signal Intensity
- PMID: 29943081
- PMCID: PMC6087495
- DOI: 10.1007/s13361-018-1994-y
Variation in FPOP Measurements Is Primarily Caused by Poor Peptide Signal Intensity
Abstract
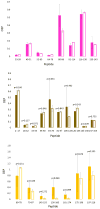
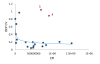
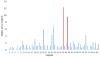
Fast photochemical oxidation of proteins (FPOP) may be used to characterize changes in protein structure by measuring differences in the apparent rate of peptide oxidation by hydroxyl radicals. The variability between replicates is high for some peptides and limits the statistical power of the technique, even using modern methods controlling variability in radical dose and quenching. Currently, the root cause of this variability has not been systematically explored, and it is unknown if the major source(s) of variability are structural heterogeneity in samples, remaining irreproducibility in FPOP oxidation, or errors in LC-MS quantification of oxidation. In this work, we demonstrate that coefficient of variation of FPOP measurements varies widely at low peptide signal intensity, but stabilizes to ≈ 0.13 at higher peptide signal intensity. We dramatically reduced FPOP variability by increasing the total sample loaded onto the LC column, indicating that the major source of variability in FPOP measurements is the difficulties in quantifying oxidation at low peptide signal intensities. This simple method greatly increases the sensitivity of FPOP structural comparisons, an important step in applying the technique to study subtle conformational changes and protein-ligand interactions. Graphical Abstract ᅟ.
Keywords: FPOP; Hydroxyl radical protein footprinting; Protein oxidation.
Figures

References
-
- Sharp JS, Becker JM, Hettich RL. Protein surface mapping by chemical oxidation: structural analysis by mass spectrometry. Anal Biochem. 2003;313(2):216–225. - PubMed
-
- Vahidi S, Stocks BB, Liaghati-Mobarhan Y, Konermann L. Mapping pH-Induced Protein Structural Changes Under Equilibrium Conditions by Pulsed Oxidative Labeling and Mass Spectrometry. Anal Chem. 2012;84(21):9124–9130. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

