Immune checkpoint blockade therapy of mesothelioma: a clinical and radiological challenge
- PMID: 29943157
- PMCID: PMC11028073
- DOI: 10.1007/s00262-018-2191-3
Immune checkpoint blockade therapy of mesothelioma: a clinical and radiological challenge
Abstract
Treatment of malignant pleural mesothelioma (MPM) represents a highly unmet medical need. Here, we discuss the results and therapeutic potential of first- and second-generation immunomodulatory antibodies targeting distinct immune checkpoints for the treatment of MPM, as well as their prospective therapeutic role in combination strategies. We also discuss the role of appropriate radiological criteria of response for MPM and the potential need of ad hoc criteria of disease evaluation in MPM patients undergoing treatment with immunotherapeutic agents.
Keywords: Immune checkpoint; Immunotherapy; Mesothelioma; NIBIT-2016.
Conflict of interest statement
Luana Calabrò served on the advisory board of Bristol Myers Squibb; Aldo Morra declares no conflict of interest; Robin Cornelissen served on advisory boards of Boehringer-Ingelheim, Roche, and Lilly; Joachim Aerts served on advisory boards of Eli-Lilly Bristol Myers Squibb, Roche-Genetech, Astra-Zeneca, MSD Sharp & Dohme, and Boehringer Ingelheim, and is a stock owner of Amphera Immunotherapy; Michele Maio served on advisory boards of Bristol Myers Squibb, Roche-Genentech, and AstraZeneca-MedImmune.
Figures
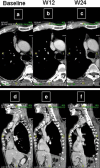

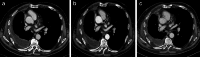
References
-
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. - DOI - PubMed
-
- Zalcman G, Mazieres J, Margery J, Greillier R, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, Rivièr F, Janicot H, Gervais R, Locher C, Milleron B, Tran Q, Lebitasy MP, Morin F, Creveuil C, Parienti JJ, Scherpereel A, French Cooperative Thoracic Intergroup (IFCT) Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405–1414. doi: 10.1016/S0140-6736(15)01238-6. - DOI - PubMed
-
- Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, Öhman R, Plummer R, Eberhardt WE, Fukuoka K, Gaafar RM, Lafitte JJ, Hillerdal G, Chu Q, Buikhuisen WA, Lubiniecki GM, Sun X, Smith M, Baas P. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015;16(4):447–456. doi: 10.1016/S1470-2045(15)70056-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

