Recurrent Glioblastoma Treated with Recombinant Poliovirus
- PMID: 29943666
- PMCID: PMC6065102
- DOI: 10.1056/NEJMoa1716435
Recurrent Glioblastoma Treated with Recombinant Poliovirus
Abstract
Background: The prognosis of patients with recurrent World Health Organization (WHO) grade IV malignant glioma is dismal, and there is currently no effective therapy. We conducted a dose-finding and toxicity study in this population of patients, evaluating convection-enhanced, intratumoral delivery of the recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO). PVSRIPO recognizes the poliovirus receptor CD155, which is widely expressed in neoplastic cells of solid tumors and in major components of the tumor microenvironment.
Methods: We enrolled consecutive adult patients who had recurrent supratentorial WHO grade IV malignant glioma, confirmed on histopathological testing, with measurable disease (contrast-enhancing tumor of ≥1 cm and ≤5.5 cm in the greatest dimension). The study evaluated seven doses, ranging between 107 and 1010 50% tissue-culture infectious doses (TCID50), first in a dose-escalation phase and then in a dose-expansion phase.
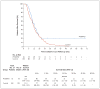
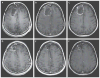
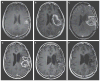
Results: From May 2012 through May 2017, a total of 61 patients were enrolled and received a dose of PVSRIPO. Dose level -1 (5.0×107 TCID50) was identified as the phase 2 dose. One dose-limiting toxic effect was observed; a patient in whom dose level 5 (1010 TCID50) was administered had a grade 4 intracranial hemorrhage immediately after the catheter was removed. To mitigate locoregional inflammation of the infused tumor with prolonged glucocorticoid use, dose level 5 was deescalated to reach the phase 2 dose. In the dose-expansion phase, 19% of the patients had a PVSRIPO-related adverse event of grade 3 or higher. Overall survival among the patients who received PVSRIPO reached a plateau of 21% (95% confidence interval, 11 to 33) at 24 months that was sustained at 36 months.
Conclusions: Intratumoral infusion of PVSRIPO in patients with recurrent WHO grade IV malignant glioma confirmed the absence of neurovirulent potential. The survival rate among patients who received PVSRIPO immunotherapy was higher at 24 and 36 months than the rate among historical controls. (Funded by the Brain Tumor Research Charity and others; ClinicalTrials.gov number, NCT01491893 .).
Figures
Comment in
-
Exploiting Viruses to Treat Diseases.N Engl J Med. 2018 Jul 12;379(2):194-196. doi: 10.1056/NEJMe1807181. Epub 2018 Jun 26. N Engl J Med. 2018. PMID: 29943655 No abstract available.
-
Bright Future for Novel Viral Glioblastoma Multiforme Therapy.World Neurosurg. 2018 Sep;117:463. doi: 10.1016/j.wneu.2018.07.113. Epub 2018 Jul 18. World Neurosurg. 2018. PMID: 30031187 No abstract available.
-
Modified Poliovirus Tested in Glioblastoma.Cancer Discov. 2018 Sep;8(9):1053-1054. doi: 10.1158/2159-8290.CD-NB2018-098. Epub 2018 Jul 24. Cancer Discov. 2018. PMID: 30042118
-
Viruses in cancer therapy - from benchwarmers to quarterbacks.Nat Rev Clin Oncol. 2018 Nov;15(11):657-658. doi: 10.1038/s41571-018-0077-0. Nat Rev Clin Oncol. 2018. PMID: 30057404 Free PMC article.
References
-
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. - PubMed
-
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–43. - PubMed
-
- Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–53. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
