Exploration of the Effects of γ-Phosphate-Modified ATP Analogues on Histidine Kinase Autophosphorylation
- PMID: 29944360
- PMCID: PMC6402838
- DOI: 10.1021/acs.biochem.8b00485
Exploration of the Effects of γ-Phosphate-Modified ATP Analogues on Histidine Kinase Autophosphorylation
Abstract
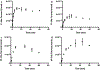
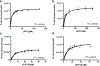
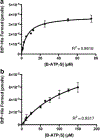
While two-component systems (TCSs), composed of a sensor histidine kinase (HK) and a response regulator, are the main signaling pathways in bacteria, global TCS activity remains poorly described. Here, we report the kinetic parameters of the HK autophosphorylation reaction using previously uncharacterized γ-phosphate-modified ATP analogues to further elucidate their utility as activity-based probes for global TCS analysis. Given the increased stability of thiophosphorylated histidine in comparison to that of the native phosphoryl modification, which is attributed to the decreased electrophilicity of this moiety, we anticipated that ATPγS may be turned over much more slowly by the HKs. Surprisingly, we found this not to be the case, with the turnover numbers decreasing <1 order of magnitude. Instead, we found that alkylation of the thiophosphate had a much more dramatic effect on turnover and, in one case, the binding affinity of this substrate analogue (BODIPY-FL-ATPγS).
Figures

Similar articles
-
Rational modulation of the enzymatic intermediates for tuning the phosphatase activity of histidine kinase HK853.Biochem Biophys Res Commun. 2020 Mar 12;523(3):733-738. doi: 10.1016/j.bbrc.2020.01.036. Epub 2020 Jan 14. Biochem Biophys Res Commun. 2020. PMID: 31948765
-
Activation of Bacterial Histidine Kinases: Insights into the Kinetics of the cis Autophosphorylation Mechanism.mSphere. 2018 May 16;3(3):e00111-18. doi: 10.1128/mSphere.00111-18. eCollection 2018 May-Jun. mSphere. 2018. PMID: 29769379 Free PMC article.
-
Kinetic and mechanistic analyses of new classes of inhibitors of two-component signal transduction systems using a coupled assay containing HpkA-DrrA from Thermotoga maritima.Microbiology (Reading). 2004 Apr;150(Pt 4):885-896. doi: 10.1099/mic.0.26824-0. Microbiology (Reading). 2004. PMID: 15073298
-
Atypical modes of bacterial histidine kinase signaling.Mol Microbiol. 2017 Jan;103(2):197-202. doi: 10.1111/mmi.13525. Epub 2016 Sep 30. Mol Microbiol. 2017. PMID: 27618209 Free PMC article. Review.
-
Recent advances in the Phos-tag technique focused on the analysis of phosphoproteins in a bacterial two-component system.J Proteomics. 2022 Feb 10;252:104429. doi: 10.1016/j.jprot.2021.104429. Epub 2021 Nov 20. J Proteomics. 2022. PMID: 34813946 Review.
Cited by
-
Chemoproteomic Approaches for Unraveling Prokaryotic Biology.Isr J Chem. 2023 Mar;63(3-4):e202200076. doi: 10.1002/ijch.202200076. Epub 2023 Feb 28. Isr J Chem. 2023. PMID: 37842282 Free PMC article.
-
Metal Messengers: Communication in the Bacterial World through Transition-Metal-Sensing Two-Component Systems.Biochemistry. 2023 Aug 15;62(16):2339-2357. doi: 10.1021/acs.biochem.3c00296. Epub 2023 Aug 4. Biochemistry. 2023. PMID: 37539997 Free PMC article. Review.
-
Immunoblot-based activity assay for heme-containing histidine kinases.bioRxiv [Preprint]. 2025 May 30:2025.05.28.656737. doi: 10.1101/2025.05.28.656737. bioRxiv. 2025. PMID: 40501847 Free PMC article. Preprint.
-
Mechanistic Studies of Bioorthogonal ATP Analogues for Assessment of Histidine Kinase Autophosphorylation.ACS Chem Biol. 2020 May 15;15(5):1252-1260. doi: 10.1021/acschembio.9b01024. Epub 2020 Feb 24. ACS Chem Biol. 2020. PMID: 32043868 Free PMC article.
-
The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing.J Fungi (Basel). 2021 May 17;7(5):393. doi: 10.3390/jof7050393. J Fungi (Basel). 2021. PMID: 34067802 Free PMC article.
References
-
- Hong HJ, Hutchings MI, and Buttner MJ (2008) Biotechnology and Biological Sciences Research Council, U.K., Vancomycin resistance VanS/VanR two-component systems. Adv. Exp. Med. Biol. 631, 200–213. - PubMed
-
- Hong HJ, Hutchings MI, Hill LM, and Buttner MJ (2005) The role of the novel Fem protein VanK in vancomycin resistance in Streptomyces coelicolor. J. Biol. Chem. 280, 13055–13061. - PubMed
-
- Prost LR, and Miller SI (2008) The Salmonellae PhoQ sensor: mechanisms of detection of phagosome signals. Cell. Microbiol. 10, 576–582. - PubMed
-
- Stock AM, Robinson VL, and Goudreau PN (2000) Two- component signal transduction. Annu. Rev. Biochem. 69, 183–215. - PubMed
-
- Stock JB, Stock AM, and Mottonen JM (1990) Signal transduction in bacteria. Nature 344, 395–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

