Identification of Morpholino Thiophenes as Novel Mycobacterium tuberculosis Inhibitors, Targeting QcrB
- PMID: 29944372
- PMCID: PMC6089501
- DOI: 10.1021/acs.jmedchem.8b00172
Identification of Morpholino Thiophenes as Novel Mycobacterium tuberculosis Inhibitors, Targeting QcrB
Abstract
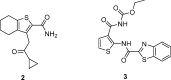
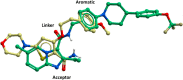
With the emergence of multidrug-resistant strains of Mycobacterium tuberculosis there is a pressing need for new oral drugs with novel mechanisms of action. Herein, we describe the identification of a novel morpholino-thiophenes (MOT) series following phenotypic screening of the Eli Lilly corporate library against M. tuberculosis strain H37Rv. The design, synthesis, and structure-activity relationships of a range of analogues around the confirmed actives are described. Optimized leads with potent whole cell activity against H37Rv, no cytotoxicity flags, and in vivo efficacy in an acute murine model of infection are described. Mode-of-action studies suggest that the novel scaffold targets QcrB, a subunit of the menaquinol cytochrome c oxidoreductase, part of the bc1-aa3-type cytochrome c oxidase complex that is responsible for driving oxygen-dependent respiration.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Global tuberculosis report 2015; World Health Organization: Geneva, Switzerland, 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

