Leptin regulation of core body temperature involves mechanisms independent of the thyroid axis
- PMID: 29944392
- PMCID: PMC6230702
- DOI: 10.1152/ajpendo.00462.2017
Leptin regulation of core body temperature involves mechanisms independent of the thyroid axis
Abstract
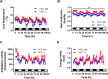
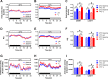
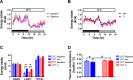
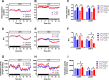
The ability to maintain core temperature within a narrow range despite rapid and dramatic changes in environmental temperature is essential for the survival of free-living mammals, and growing evidence implicates an important role for the hormone leptin. Given that thyroid hormone plays a major role in thermogenesis and that circulating thyroid hormone levels are reduced in leptin-deficient states (an effect partially restored by leptin replacement), we sought to determine the extent to which leptin's role in thermogenesis is mediated by raising thyroid hormone levels. To this end, we 1) quantified the effect of physiological leptin replacement on circulating levels of thyroid hormone in leptin-deficient ob/ob mice, and 2) determined if the effect of leptin to prevent the fall in core temperature in these animals during cold exposure is mimicked by administration of a physiological replacement dose of triiodothyronine (T3). We report that, as with leptin, normalization of circulating T3 levels is sufficient both to increase energy expenditure, respiratory quotient, and ambulatory activity and to reduce torpor in ob/ob mice. Yet, unlike leptin, infusing T3 at a dose that normalizes plasma T3 levels fails to prevent the fall of core temperature during mild cold exposure. Because thermal conductance (e.g., heat loss to the environment) was reduced by administration of leptin but not T3, leptin regulation of heat dissipation is implicated as playing a uniquely important role in thermoregulation. Together, these findings identify a key role in thermoregulation for leptin-mediated suppression of thermal conduction via a mechanism that is independent of the thyroid axis.
Keywords: body temperature; energy expenditure; energy intake; leptin; thermal conductance; thermoregulation; thyroid hormone.
Figures





Similar articles
-
Leptin signaling is required for adaptive changes in food intake, but not energy expenditure, in response to different thermal conditions.PLoS One. 2015 Mar 10;10(3):e0119391. doi: 10.1371/journal.pone.0119391. eCollection 2015. PLoS One. 2015. PMID: 25756181 Free PMC article.
-
Physiological role for leptin in the control of thermal conductance.Mol Metab. 2016 Jul 20;5(10):892-902. doi: 10.1016/j.molmet.2016.07.005. eCollection 2016 Oct. Mol Metab. 2016. PMID: 27689002 Free PMC article.
-
Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress.Endocrinology. 2006 May;147(5):2468-80. doi: 10.1210/en.2005-1216. Epub 2006 Feb 9. Endocrinology. 2006. PMID: 16469807
-
Integration of sensory information via central thermoregulatory leptin targets.Physiol Behav. 2013 Sep 10;121:49-55. doi: 10.1016/j.physbeh.2013.02.014. Epub 2013 Feb 28. Physiol Behav. 2013. PMID: 23458626 Free PMC article. Review.
-
Effects of thermal stress during rest and exercise in the paediatric population.Sports Med. 1998 Apr;25(4):221-40. doi: 10.2165/00007256-199825040-00002. Sports Med. 1998. PMID: 9587181 Review.
Cited by
-
Fibroblast Growth Factor-21, Leptin, and Adiponectin Responses to Acute Cold-Induced Brown Adipose Tissue Activation.J Clin Endocrinol Metab. 2020 Mar 1;105(3):e520-31. doi: 10.1210/clinem/dgaa005. J Clin Endocrinol Metab. 2020. PMID: 31912874 Free PMC article.
-
Validation of an equation for energy expenditure that does not require the respiratory quotient.PLoS One. 2019 Feb 1;14(2):e0211585. doi: 10.1371/journal.pone.0211585. eCollection 2019. PLoS One. 2019. PMID: 30707737 Free PMC article.
-
Thermally induced neuronal plasticity in the hypothalamus mediates heat tolerance.Nat Neurosci. 2025 Feb;28(2):346-360. doi: 10.1038/s41593-024-01830-0. Epub 2024 Dec 9. Nat Neurosci. 2025. PMID: 39653806 Free PMC article.
-
Leptin: Is It Thermogenic?Endocr Rev. 2020 Apr 1;41(2):232-60. doi: 10.1210/endrev/bnz016. Endocr Rev. 2020. PMID: 31774114 Free PMC article. Review.
-
Rats that are predisposed to excessive obesity show reduced (leptin-induced) thermoregulation even in the preobese state.Physiol Rep. 2019 Jul;7(14):e14102. doi: 10.14814/phy2.14102. Physiol Rep. 2019. PMID: 31342663 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

