The clinical, immunological and microbiological impact of the 10-valent pneumococcal-Protein D conjugate vaccine in children with recurrent protracted bacterial bronchitis, chronic suppurative lung disease and bronchiectasis: A multi-centre, double-blind, randomised controlled trial
- PMID: 29944440
- PMCID: PMC6314404
- DOI: 10.1080/21645515.2018.1488562
The clinical, immunological and microbiological impact of the 10-valent pneumococcal-Protein D conjugate vaccine in children with recurrent protracted bacterial bronchitis, chronic suppurative lung disease and bronchiectasis: A multi-centre, double-blind, randomised controlled trial
Abstract
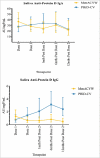
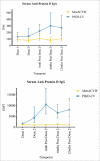
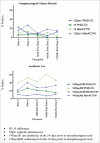
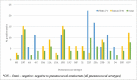
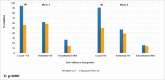
We aimed to determine the efficacy of the 10-valent pneumococcal-Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in children aged 18-months to <18-years with recurrent protracted bacterial bronchitis (rPBB), chronic suppurative lung disease (CSLD) or bronchiectasis. In a multi-centre, double-blind randomised controlled trial, children received two doses, 2-months apart of the 10vPHiD-CV or quadrivalent meningococcal-ACYW135 conjugate vaccine. Active surveillance for acute exacerbations, respiratory symptoms and antibiotic use was undertaken through to 12-months after the second vaccine dose (clinical cohort only). Serum, saliva and nasopharyngeal swabs were collected to measure immunological and microbiological effects (immunology cohort). Between December 2012 and August 2015, 62 children were enrolled onto the clinical protocol (1 excluded from clinical analyses due to unblinding), while 74 contributed to the immunology cohort (overall mean age = 6.8-years (standard deviation = 3.7), 42 (56.8%) male). The absolute risk difference comparing the 10vPHiD-CV group (n = 31 children) to the MenACYW135 group (n = 30 children) for acute exacerbations was -0.5 exacerbations/100-weeks at risk (95% confidence interval (CI) -2.0, 0.9). Compared to the MenACYW135 group, children who received the 10vPHiD-CV were less likely to have respiratory symptoms in each fortnight of surveillance (incidence density ratio (IDR) 0.82, 95%CI 0.61, 1.10) and required fewer short-course (<14-days duration) antibiotics (IDR 0.81, 95% CI 0.61, 1.09). The vaccine was immunogenic and no serious adverse events related to the vaccine were reported. In conclusion, 10vPHiD-CV might have a future role in managing children with rPBB, CSLD and bronchiectasis, but larger multicentre trials are needed to confirm or refute findings from this preliminary study.
Keywords: H. influenzae; PHID-CV; acute exacerbation; bronchiectasis; children; chronic suppurative lung disease; efficacy; prevention; protracted bacterial bronchitis.
Figures
Similar articles
-
Does a 10-valent pneumococcal-Haemophilus influenzae protein D conjugate vaccine prevent respiratory exacerbations in children with recurrent protracted bacterial bronchitis, chronic suppurative lung disease and bronchiectasis: protocol for a randomised controlled trial.Trials. 2013 Sep 5;14:282. doi: 10.1186/1745-6215-14-282. Trials. 2013. PMID: 24010917 Free PMC article. Clinical Trial.
-
Reduced nontypeable Haemophilus influenzae lower airway infection in children with chronic endobronchial suppuration vaccinated with the 10-valent pneumococcal H. influenzae protein D conjugate vaccine.Vaccine. 2018 Mar 20;36(13):1736-1742. doi: 10.1016/j.vaccine.2018.02.054. Epub 2018 Feb 23. Vaccine. 2018. PMID: 29478754
-
Safety, reactogenicity and immunogenicity of 2-dose catch-up vaccination with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Malian children in the second year of life: Results from an open study.Hum Vaccin Immunother. 2015;11(9):2207-14. doi: 10.1080/21645515.2015.1016679. Hum Vaccin Immunother. 2015. PMID: 26020101 Free PMC article. Clinical Trial.
-
Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines.Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S109-18. doi: 10.1097/INF.0b013e318199f62d. Pediatr Infect Dis J. 2009. PMID: 19325447 Review.
-
Streptococcus pneumoniae and chronic endobronchial infections in childhood.Pediatr Pulmonol. 2017 Dec;52(12):1532-1545. doi: 10.1002/ppul.23828. Epub 2017 Sep 18. Pediatr Pulmonol. 2017. PMID: 28922566 Review.
Cited by
-
Australia's Role in Pneumococcal and Human Papillomavirus Vaccine Evaluation in Asia-Pacific.Vaccines (Basel). 2021 Aug 18;9(8):921. doi: 10.3390/vaccines9080921. Vaccines (Basel). 2021. PMID: 34452046 Free PMC article. Review.
-
Effect of vaccination on the use of antimicrobial agents: a systematic literature review.Ann Med. 2020 Sep;52(6):283-299. doi: 10.1080/07853890.2020.1782460. Epub 2020 Jun 29. Ann Med. 2020. PMID: 32597236 Free PMC article.
-
Update on protracted bacterial bronchitis in children.Ital J Pediatr. 2020 Mar 30;46(1):38. doi: 10.1186/s13052-020-0802-z. Ital J Pediatr. 2020. PMID: 32228653 Free PMC article.
-
The impact of influenza and pneumococcal vaccination on antibiotic use: an updated systematic review and meta-analysis.Antimicrob Resist Infect Control. 2023 Jul 14;12(1):70. doi: 10.1186/s13756-023-01272-6. Antimicrob Resist Infect Control. 2023. PMID: 37452389 Free PMC article.
-
Interchangeability, immunogenicity and safety of a combined 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (Synflorix) and 13-valent-PCV (Prevenar13) schedule at 1-2-4-6 months: PREVIX_COMBO, a 3-arm randomised controlled trial.Vaccine X. 2021 Feb 15;7:100086. doi: 10.1016/j.jvacx.2021.100086. eCollection 2021 Apr. Vaccine X. 2021. PMID: 33681756 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
