Modeling Cell and Tumor-Metastasis Dosimetry with the Particle and Heavy Ion Transport Code System (PHITS) Software for Targeted Alpha-Particle Radionuclide Therapy
- PMID: 29944461
- PMCID: PMC6512332
- DOI: 10.1667/RR15081.1
Modeling Cell and Tumor-Metastasis Dosimetry with the Particle and Heavy Ion Transport Code System (PHITS) Software for Targeted Alpha-Particle Radionuclide Therapy
Abstract
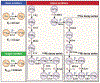
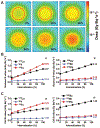
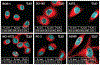
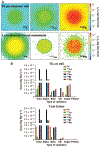
The use of targeted radionuclide therapy for cancer is on the rise. While beta-particle-emitting radionuclides have been extensively explored for targeted radionuclide therapy, alpha-particle-emitting radionuclides are emerging as effective alternatives. In this context, fundamental understanding of the interactions and dosimetry of these emitted particles with cells in the tumor microenvironment is critical to ascertaining the potential of alpha-particle-emitting radionuclides. One important parameter that can be used to assess these metrics is the S-value. In this study, we characterized several alpha-particle-emitting radionuclides (and their associated radionuclide progeny) regarding S-values in the cellular and tumor-metastasis environments. The Particle and Heavy Ion Transport code System (PHITS) was used to obtain S-values via Monte Carlo simulation for cell and tumor metastasis resulting from interactions with the alpha-particle-emitting radionuclides, lead-212 (212Pb), actinium-225 (225Ac) and bismuth-213 (213Bi); these values were compared to the beta-particle-emitting radionuclides yttrium-90 (90Y) and lutetium-177 (177Lu) and an Auger-electron-emitting radionuclide indium-111 (111In). The effect of cellular internalization on S-value was explored at increasing degree of internalization for each radionuclide. This aspect of S-value determination was further explored in a cell line-specific fashion for six different cancer cell lines based on the cell dimensions obtained by confocal microscopy. S-values from PHITS were in good agreement with MIRDcell S-values (cellular S-values) and the values found by Hindié et al. (tumor S-values). In the cellular model, 212Pb and 213Bi decay series produced S-values that were 50- to 120-fold higher than 177Lu, while 225Ac decay series analysis suggested S-values that were 240- to 520-fold higher than 177Lu. S-values arising with 100% cellular internalization were two- to sixfold higher for the nucleus when compared to 0% internalization. The tumor dosimetry model defines the relative merit of radionuclides and suggests alpha particles may be effective for large tumors as well as small tumor metastases. These results from PHITS modeling substantiate emerging evidence that alpha-particle-emitting radionuclides may be an effective alternative to beta-particle-emitting radionuclides for targeted radionuclide therapy due to preferred dose-deposition profiles in the cellular and tumor metastasis context. These results further suggest that internalization of alpha-particle-emitting radionuclides via radiolabeled ligands may increase the relative biological effectiveness of radiotherapeutics.
Figures
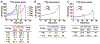
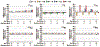
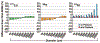
References
-
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369:213–23. - PubMed
-
- Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med 2016; 57:1170–6. - PubMed
-
- Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003; 24:389–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

