Single-dose oral ciprofloxacin prophylaxis as a response to a meningococcal meningitis epidemic in the African meningitis belt: A 3-arm, open-label, cluster-randomized trial
- PMID: 29944651
- PMCID: PMC6019097
- DOI: 10.1371/journal.pmed.1002593
Single-dose oral ciprofloxacin prophylaxis as a response to a meningococcal meningitis epidemic in the African meningitis belt: A 3-arm, open-label, cluster-randomized trial
Abstract
Background: Antibiotic prophylaxis for contacts of meningitis cases is not recommended during outbreaks in the African meningitis belt. We assessed the effectiveness of single-dose oral ciprofloxacin administered to household contacts and in village-wide distributions on the overall attack rate (AR) in an outbreak of meningococcal meningitis.
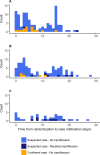
Methods and findings: In this 3-arm, open-label, cluster-randomized trial during a meningococcal meningitis outbreak in Madarounfa District, Niger, villages notifying a suspected case were randomly assigned (1:1:1) to standard care (the control arm), single-dose oral ciprofloxacin for household contacts within 24 hours of case notification, or village-wide distribution of ciprofloxacin within 72 hours of first case notification. The primary outcome was the overall AR of suspected meningitis after inclusion. A random sample of 20 participating villages was enrolled to document any changes in fecal carriage prevalence of ciprofloxacin-resistant and extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae before and after the intervention. Between April 22 and May 18, 2017, 49 villages were included: 17 to the control arm, 17 to household prophylaxis, and 15 to village-wide prophylaxis. A total of 248 cases were notified in the study after the index cases. The AR was 451 per 100,000 persons in the control arm, 386 per 100,000 persons in the household prophylaxis arm (t test versus control p = 0.68), and 190 per 100,000 persons in the village-wide prophylaxis arm (t test versus control p = 0.032). The adjusted AR ratio between the household prophylaxis arm and the control arm was 0.94 (95% CI 0.52-1.73, p = 0.85), and the adjusted AR ratio between the village-wide prophylaxis arm and the control arm was 0.40 (95% CI 0.19‒0.87, p = 0.022). No adverse events were notified. Baseline carriage prevalence of ciprofloxacin-resistant Enterobacteriaceae was 95% and of ESBL-producing Enterobacteriaceae was >90%, and did not change post-intervention. One limitation of the study was the small number of cerebrospinal fluid samples sent for confirmatory testing.
Conclusions: Village-wide distribution of single-dose oral ciprofloxacin within 72 hours of case notification reduced overall meningitis AR. Distributions of ciprofloxacin could be an effective tool in future meningitis outbreak responses, but further studies investigating length of protection, effectiveness in urban settings, and potential impact on antimicrobial resistance patterns should be carried out.
Trial registration: ClinicalTrials.gov NCT02724046.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: RFG is a member of the Editorial Board of PLOS Medicine.
Figures
Comment in
-
Antibiotic prophylaxis-Preventing severe infections and saving lives in poor countries with very high mortality risk.PLoS Med. 2018 Jun 26;15(6):e1002594. doi: 10.1371/journal.pmed.1002594. eCollection 2018 Jun. PLoS Med. 2018. PMID: 29944649 Free PMC article.
Similar articles
-
Analysis of a meningococcal meningitis outbreak in Niger - potential effectiveness of reactive prophylaxis.PLoS Negl Trop Dis. 2019 Mar 11;13(3):e0007077. doi: 10.1371/journal.pntd.0007077. eCollection 2019 Mar. PLoS Negl Trop Dis. 2019. PMID: 30856166 Free PMC article.
-
Ciprofloxacin for contacts of cases of meningococcal meningitis as an epidemic response: study protocol for a cluster-randomized trial.Trials. 2017 Jun 24;18(1):294. doi: 10.1186/s13063-017-2028-y. Trials. 2017. PMID: 28646924 Free PMC article. Clinical Trial.
-
Sequelae following an epidemic of meningococcal meningitis in Niger in 2022.PLoS One. 2025 May 22;20(5):e0323223. doi: 10.1371/journal.pone.0323223. eCollection 2025. PLoS One. 2025. PMID: 40403034 Free PMC article.
-
Meningococcal carriage by age in the African meningitis belt: a systematic review and meta-analysis.Epidemiol Infect. 2019 Jan;147:e228. doi: 10.1017/S0950268819001134. Epidemiol Infect. 2019. PMID: 31364554 Free PMC article.
-
Priorities for research on meningococcal disease and the impact of serogroup A vaccination in the African meningitis belt.Vaccine. 2013 Mar 1;31(11):1453-7. doi: 10.1016/j.vaccine.2012.12.035. Epub 2012 Dec 27. Vaccine. 2013. PMID: 23273967 Free PMC article.
Cited by
-
Recent Evolution of Susceptibility to Beta-Lactams in Neisseria meningitidis.Antibiotics (Basel). 2023 Jun 1;12(6):992. doi: 10.3390/antibiotics12060992. Antibiotics (Basel). 2023. PMID: 37370311 Free PMC article.
-
Antibiotic resistance among invasive Neisseria meningitidis isolates in England, Wales and Northern Ireland (2010/11 to 2018/19).PLoS One. 2021 Nov 29;16(11):e0260677. doi: 10.1371/journal.pone.0260677. eCollection 2021. PLoS One. 2021. PMID: 34843604 Free PMC article.
-
Analysis of a meningococcal meningitis outbreak in Niger - potential effectiveness of reactive prophylaxis.PLoS Negl Trop Dis. 2019 Mar 11;13(3):e0007077. doi: 10.1371/journal.pntd.0007077. eCollection 2019 Mar. PLoS Negl Trop Dis. 2019. PMID: 30856166 Free PMC article.
-
Inhibitory Concentrations of Ciprofloxacin Induce an Adaptive Response Promoting the Intracellular Survival of Salmonella enterica Serovar Typhimurium.mBio. 2021 Jun 29;12(3):e0109321. doi: 10.1128/mBio.01093-21. Epub 2021 Jun 22. mBio. 2021. PMID: 34154399 Free PMC article.
-
Molecular detection of fluoroquinolone-resistant Neisseria meningitidis by using mismatched PCR-restriction fragment length polymorphism technique.Front Cell Infect Microbiol. 2022 Aug 2;12:911911. doi: 10.3389/fcimb.2022.911911. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35982783 Free PMC article.
References
-
- Lapeyssonnie L. La méningite cérébrospinale en Afrique. Bull WHO. 1963;28:53–114. - PubMed
-
- Greenwood BM. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;43:341–53. doi: 10.1016/S0035-9203(99)90106-2 - DOI - PubMed
-
- Boisier P, Nicolas P, Djibo S, Taha M-K, Jeanne I, Maïnassara HB, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646 - DOI - PubMed
-
- Nathan N, Rose AMC, Legros D, Tiendrebeogo SRM, Bachy C, Bjørløw E, et al. Meningitis serogroup W135 outbreak, Burkina Faso, 2002. Emerg Infect Dis. 2007;13:920–3. doi: 10.3201/eid1306.060940 - DOI - PMC - PubMed
-
- Collard J-M, Maman Z, Yacouba H, Djibo S, Nicolas P, Jusot J-F, et al. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg Infect Dis. 2010;16:1496–8. doi: 10.3201/eid1609.100510 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous