Corticosteroid inhibits differentiation of palmar fibromatosis-derived stem cells (FSCs) through downregulation of transforming growth factor-β1 (TGF-β1)
- PMID: 29944666
- PMCID: PMC6019676
- DOI: 10.1371/journal.pone.0198326
Corticosteroid inhibits differentiation of palmar fibromatosis-derived stem cells (FSCs) through downregulation of transforming growth factor-β1 (TGF-β1)
Abstract
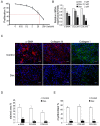
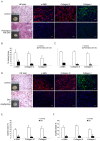
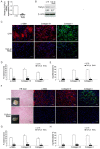
Treatment for musculoskeletal fibromatosis remains challenging. Surgical excision for fibromatosis is the standard therapy but recurrence remains high. Corticosteroids, an anti-fibrogenic compound, have been used to treat early stage palmar fibromatosis, but the mechanism is unknown. We investigated the inhibitory mechanism effect of corticosteroids in the murine model of fibromatosis nodule as well as in cultured FSCs. Quantitative reverse transcription/polymerase chain reaction (PCR) analysis and immunofluorescence (IF) staining for markers of myofibroblasts (α-smooth muscle actin and type III collagen) were used to examine the effect of dexamethasone on myofibroblasic differentiation of FSCs both in vitro and in vivo. Transforming growth factor-β1 (TGF-β1) signaling and its downstream targets were examined using western blot analysis. TGF-β1 expression in FSCs before and after dexamethasone treatment was compared. In addition, inhibition of TGF-β1 expression was examined using RNA interference (RNAi) on FSCs, both in vitro and in vivo. Treating FSCs with dexamethasone inhibited FSCs' myofibroblastic differentiation in vitro. Treating FSCs with dexamethasone before or after implantation further inhibited formation of fibromatosis nodules. Dexamethasone suppressed expression of TGF-β1 and pSmad2/3 by FSCs in vitro. TGF-β1 knockdown FSCs showed reducing myofibroblastic differentiation both in vitro and in vivo. Finally, addition of TGF-β1 abolished dexamethasone-mediated inhibition of myofibroblastic differentiation. Dexamethasone inhibits the myofibroblastic differentiated potential of FSCs both in vitro and in vivo through inhibition of TGF-β1 expression in FSCs. TGF-β1 plays a key role in myofibroblastic differentiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Fibromatosis stem cells rather than bone-marrow mesenchymal stem cells recapitulate a murine model of fibromatosis.Biochem Biophys Res Commun. 2011 May 6;408(2):269-75. doi: 10.1016/j.bbrc.2011.04.011. Epub 2011 Apr 8. Biochem Biophys Res Commun. 2011. PMID: 21501590
-
Recapitulation of fibromatosis nodule by multipotential stem cells in immunodeficient mice.PLoS One. 2011;6(8):e24050. doi: 10.1371/journal.pone.0024050. Epub 2011 Aug 25. PLoS One. 2011. PMID: 21901157 Free PMC article.
-
Notch2 negatively regulates myofibroblastic differentiation of myoblasts.J Cell Physiol. 2007 Feb;210(2):358-69. doi: 10.1002/jcp.20838. J Cell Physiol. 2007. PMID: 17044085
-
The Basic Science of Dupuytren Disease.Hand Clin. 2018 Aug;34(3):301-305. doi: 10.1016/j.hcl.2018.03.001. Epub 2018 Jun 8. Hand Clin. 2018. PMID: 30012290 Review.
-
Palmar and plantar fibromatosis: a review.J Pathol Transl Med. 2021 Jul;55(4):265-270. doi: 10.4132/jptm.2021.06.14. Epub 2021 Jul 7. J Pathol Transl Med. 2021. PMID: 34225446 Free PMC article. Review.
Cited by
-
Current Strategies in Assessment of Nanotoxicity: Alternatives to In Vivo Animal Testing.Int J Mol Sci. 2021 Apr 19;22(8):4216. doi: 10.3390/ijms22084216. Int J Mol Sci. 2021. PMID: 33921715 Free PMC article. Review.
-
Comprehensive insights into mechanism of nanotoxicity, assessment methods and regulatory challenges of nanomedicines.Discov Nano. 2024 Oct 4;19(1):165. doi: 10.1186/s11671-024-04118-1. Discov Nano. 2024. PMID: 39365367 Free PMC article. Review.
-
Network Meta-Analysis of Different Clinical Commonly Used Drugs for the Treatment of Hypertrophic Scar and Keloid.Front Med (Lausanne). 2021 Sep 9;8:691628. doi: 10.3389/fmed.2021.691628. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34568361 Free PMC article.
-
Cymbopogon winterianus Essential Oil Attenuates Bleomycin-Induced Pulmonary Fibrosis in a Murine Model.Pharmaceutics. 2021 May 9;13(5):679. doi: 10.3390/pharmaceutics13050679. Pharmaceutics. 2021. PMID: 34065064 Free PMC article.
-
A case of papilledema in Camurati-Engelmann disease treated effectively with prednisolone.Clin Pediatr Endocrinol. 2023;32(3):174-179. doi: 10.1297/cpe.2023-0009. Epub 2023 Apr 28. Clin Pediatr Endocrinol. 2023. PMID: 37362159 Free PMC article.
References
-
- Bayat A, Cunliffe EJ, McGrouther DA: Assessment of clinical severity in Dupuytren’s disease. Br J Hosp Med 2007; 68:604–609. doi: 10.12968/hmed.2007.68.11.27683 . - DOI - PubMed
-
- Wang JP, Hui YJ, Wang ST, Huang YC, Chiang ER, Liu CL, et al.: Fibromatosis stem cells rather than bone-marrow mesenchymal stem cells recapitulate a murine model of fibromatosis. Biochem Biophys Res Commu 2011; 408:269–275. doi: 10.1016/j.bbrc.2011.04.011 . - DOI - PubMed
-
- Wang JP, Hui YJ, Wang ST, Yu HH, Huang YC, Chiang ER, et al.: Recapitulation of fibromatosis nodule by multipotential stem cells in immunodeficient mice. PLoS ONE 2011; 6:e24050 doi: 10.1371/journal.pone.0024050 . - DOI - PMC - PubMed
-
- Le Poole IC, Boyce ST: Keratinocytes suppress transforming growth factor-beta1 expression by fibroblasts in cultured skin substitutes. Br J Dermatol 1999; 140:409–416. . - PubMed
-
- Mauviel A: Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med 2005; 117:69–80. doi: 10.1385/1-59259-940-0:069 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

