Metabolites of n-Butylparaben and iso-Butylparaben Exhibit Estrogenic Properties in MCF-7 and T47D Human Breast Cancer Cell Lines
- PMID: 29945225
- PMCID: PMC6016648
- DOI: 10.1093/toxsci/kfy063
Metabolites of n-Butylparaben and iso-Butylparaben Exhibit Estrogenic Properties in MCF-7 and T47D Human Breast Cancer Cell Lines
Abstract
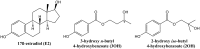
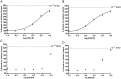
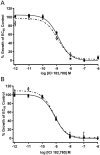
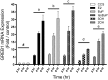
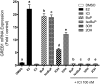
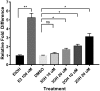
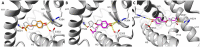
Two oxidized metabolites of n-butylparaben (BuP) and iso-butylparaben (IsoBuP) discovered in human urine samples exhibit structural similarity to endogenous estrogens. We hypothesized that these metabolites bind to the human estrogen receptor (ER) and promote estrogen signaling. We tested this using models of ER-mediated cellular proliferation. The estrogenic properties of 3-hydroxy n-butyl 4-hydroxybenzoate (3OH) and 2-hydroxy iso-butyl 4-hydroxybenzoate (2OH) were determined using the ER-positive, estrogen-dependent human breast cancer cell lines MCF-7, and T47D. The 3OH metabolite induced cellular proliferation with EC50 of 8.2 µM in MCF-7 cells. The EC50 for 3OH in T47D cells could not be reached. The 2OH metabolite induced proliferation with EC50 of 2.2 µM and 43.0 µM in MCF-7 and T47D cells, respectively. The EC50 for the parental IsoBuP and BuP was 0.30 and 1.2 µM in MCF-7 cells, respectively. The expression of a pro-proliferative, estrogen-inducible gene (GREB1) was induced by these compounds and blocked by co-administration of an ER antagonist (ICI 182, 780), confirming the ER-dependence of these effects. The metabolites promoted significant ER-dependent transcriptional activity of an ERE-luciferase reporter construct at 10 and 20 µM for 2OH and 10 µM for 3OH. Computational docking studies showed that the paraben compounds exhibited the potential for favorable ligand-binding domain interactions with human ERα in a manner similar to known x-ray crystal structures of 17ß-estradiol in complex with ERα. We conclude that the hydroxylated metabolites of BuP and IsoBuP are weak estrogens and should be considered as additional components of potential endocrine disrupting effects upon paraben exposure.
Figures
Similar articles
-
Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage.Arch Toxicol. 2016 Nov;90(11):2699-2709. doi: 10.1007/s00204-015-1636-0. Epub 2015 Nov 25. Arch Toxicol. 2016. PMID: 26608183
-
Estrogenic and anti-estrogenic activity of butylparaben, butylated hydroxyanisole, butylated hydroxytoluene and propyl gallate and their binary mixtures on two estrogen responsive cell lines (T47D-Kbluc, MCF-7).J Appl Toxicol. 2018 Jul;38(7):944-957. doi: 10.1002/jat.3601. Epub 2018 Feb 19. J Appl Toxicol. 2018. PMID: 29460325
-
Bisphenol AF stimulates transcription and secretion of C-X-C chemokine ligand 12 to promote proliferation of cultured T47D breast cancer cells.Toxicology. 2015 Dec 2;338:30-6. doi: 10.1016/j.tox.2015.09.007. Epub 2015 Oct 3. Toxicology. 2015. PMID: 26435001
-
Inhibitory effect of silver nanoparticles on proliferation of estrogen-dependent MCF-7/BUS human breast cancer cells induced by butyl paraben or di-n-butyl phthalate.Toxicol Appl Pharmacol. 2017 Dec 15;337:12-21. doi: 10.1016/j.taap.2017.10.014. Epub 2017 Oct 23. Toxicol Appl Pharmacol. 2017. PMID: 29074358
-
A review of the endocrine activity of parabens and implications for potential risks to human health.Crit Rev Toxicol. 2005 Jun;35(5):435-58. doi: 10.1080/10408440490920104. Crit Rev Toxicol. 2005. PMID: 16097138 Review.
Cited by
-
Endocrine-Disrupting Chemicals and Breast Cancer: Disparities in Exposure and Importance of Research Inclusivity.Endocrinology. 2022 May 1;163(5):bqac034. doi: 10.1210/endocr/bqac034. Endocrinology. 2022. PMID: 35325096 Free PMC article. Review.
-
REPRODUCTIVE TOXICOLOGY: Endocrine disruption and reproductive disorders: impacts on sexually dimorphic neuroendocrine pathways.Reproduction. 2021 Oct 5;162(5):F111-F130. doi: 10.1530/REP-20-0596. Reproduction. 2021. PMID: 33929341 Free PMC article. Review.
-
Homology models of mouse and rat estrogen receptor-α ligand-binding domain created by in silico mutagenesis of a human template: molecular docking with 17ß-estradiol, diethylstilbestrol, and paraben analogs.Comput Toxicol. 2019 May;10:1-16. doi: 10.1016/j.comtox.2018.11.003. Epub 2018 Nov 28. Comput Toxicol. 2019. PMID: 30740556 Free PMC article.
-
Implication of environmental estrogens on breast cancer treatment and progression.Toxicology. 2019 Jun 1;421:41-48. doi: 10.1016/j.tox.2019.03.014. Epub 2019 Mar 30. Toxicology. 2019. PMID: 30940549 Free PMC article. Review.
-
A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999-2014.Environ Int. 2020 Apr;137:105496. doi: 10.1016/j.envint.2020.105496. Epub 2020 Feb 26. Environ Int. 2020. PMID: 32113086 Free PMC article.
References
-
- Artacho-Cordón F., Arrebola J. P., Nielsen O., Hernández P., Skakkebaek N. E., Fernández M. F., Andersson A. M., Olea N., Frederiksen H. (2017). Assumed non-persistent environmental chemicals in human adipose tissue; matrix stability and correlation with levels measured in urine and serum. Environ. Res. 156, 120–127. - PubMed
-
- Azzouz A., Rascon A. J., Ballesteros E. (2016). Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 119, 16–26. - PubMed
-
- Barr L., Metaxas G., Harbach C. A., Savoy L. A., Darbre P. D. (2012). Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 32, 219–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

