Short-course High-dose Liposomal Amphotericin B for Human Immunodeficiency Virus-associated Cryptococcal Meningitis: A Phase 2 Randomized Controlled Trial
- PMID: 29945252
- PMCID: PMC6336908
- DOI: 10.1093/cid/ciy515
Short-course High-dose Liposomal Amphotericin B for Human Immunodeficiency Virus-associated Cryptococcal Meningitis: A Phase 2 Randomized Controlled Trial
Abstract
Background: We performed a phase 2 noninferiority trial examining the early fungicidal activity (EFA) of 3 short-course, high-dose liposomal amphotericin B (L-AmB) regimens for cryptococcal meningitis (CM) in Tanzania and Botswana.
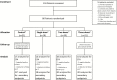
Methods: Human immunodeficiency virus (HIV)-infected adults with CM were randomized to (i) L-AmB 10 mg/kg on day 1 (single dose); (ii) L-AmB 10 mg/kg on day 1 and 5 mg/kg on day 3 (2 doses); (iii) L-AmB 10 mg/kg on day 1 and 5 mg/kg on days 3 and 7 (3 doses); or (iv) L-AmB 3 mg/kg/day for 14 days (control). All patients also received oral fluconazole 1200 mg/day for 14 days. Primary endpoint was mean rate of clearance of cerebrospinal fluid cryptococcal infection (EFA). Noninferiority was defined as an upper limit of the 2-sided 95% confidence interval (CI) of difference in EFA between intervention and control <0.2 log10 colony-forming units (CFU)/mL/day.
Results: Eighty participants were enrolled. EFA for daily L-AmB was -0.41 log10 CFU/mL/day (standard deviation, 0.11; n = 17). Difference in mean EFA from control was -0.11 (95% CI, -.29 to .07) log10 CFU/mL/day faster with single dose (n = 16); -0.05 (95% CI, -.20 to .10) log10 CFU/mL/day faster with 2 doses (n = 18); and -0.13 (95% CI, -.35 to .09) log10 CFU/mL/day faster with 3 doses (n = 18). EFA in all short-course arms was noninferior to control. Ten-week mortality was 29% (n = 23) with no statistical difference between arms. All arms were well tolerated.
Conclusions: Single-dose 10 mg/kg L-AmB was well tolerated and led to noninferior EFA compared to 14 days of 3 mg/kg/day L-AmB in HIV-associated CM. Induction based on a single 10 mg/kg L-AmB dose is being taken forward to a phase 3 clinical endpoint trial.
Clinical trials registration: ISRCTN 10248064.
Figures

References
-
- Braitstein P, Brinkhof MW, Dabis F, et al. ; Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration; ART Cohort Collaboration (ART-CC) groups Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006; 367:817–24. - PubMed
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

