Remote ischemic preconditioning for cardioprotection in elective inpatient abdominal surgery - a randomized controlled trial
- PMID: 29945555
- PMCID: PMC6020340
- DOI: 10.1186/s12871-018-0524-6
Remote ischemic preconditioning for cardioprotection in elective inpatient abdominal surgery - a randomized controlled trial
Abstract
Background: Perioperative myocardial injury (PMI) is common in elective inpatient abdominal surgery and correlates with mortality risk. Simple measures for reducing PMI in this cohort are needed. This study evaluated whether remote ischemic preconditioning (RIPC) could reduce PMI in elective inpatient abdominal surgery.
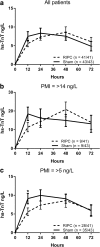
Methods: This was a double-blind, sham-controlled trial with 1:1 parallel randomization. PMI was defined as any post-operative serum troponin T (hs-TNT) > 14 ng/L. Eighty-four participants were randomized to receiving RIPC (5 min of upper arm ischemia followed by 5 min reperfusion, for three cycles) or a sham-treatment immediately prior to surgery. The primary outcome was mean peak post-operative troponin in patients with PMI, and secondary outcomes included mean hs-TnT at individual timepoints, post-operative hs-TnT area under the curve (AUC), cardiovascular events and mortality. Predictors of PMI were also collected. Follow up was to 1 year.
Results: PMI was observed in 21% of participants. RIPC did not significantly influence the mean peak post-operative hs-TnT concentration in these patients (RIPC 25.65 ng/L [SD 9.33], sham-RIPC 23.91 [SD 13.2], mean difference 1.73 ng/L, 95% confidence interval - 9.7 to 13.1 ng/L, P = 0.753). The treatment did not influence any secondary outcome with the pre-determined definition of PMI. Redefining PMI as > 5 ng/L in line with recent data revealed a non-significant lower incidence in the RIPC cohort (68% vs 81%, P = 0.211), and significantly lower early hs-TnT release (12 h time-point, RIPC 5.5 ng/L [SD 5.5] vs sham 9.1 ng/L [SD 8.2], P = 0.03).
Conclusions: RIPC did not at reduce the incidence or severity of PMI in these general surgical patients using pre-determined definitions. PMI is nonetheless common and effective cardioprotective strategies are required.
Trial registration: This trial was registered with Clinicaltrials.gov, NCT01850927 , 5th July 2013.
Keywords: General surgery; Ischemic preconditioning; Myocardial injury.
Conflict of interest statement
Ethics approval and consent to participate
The Berkshire 2 National Research Ethics Service provided ethical approval for the study (Reference: 13/SC/0306). Written informed consent was obtained from each participant prior to any data collection or study intervention.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

