Web-Based Versus Usual Care and Other Formats of Decision Aids to Support Prostate Cancer Screening Decisions: Systematic Review and Meta-Analysis
- PMID: 29945857
- PMCID: PMC6043730
- DOI: 10.2196/jmir.9070
Web-Based Versus Usual Care and Other Formats of Decision Aids to Support Prostate Cancer Screening Decisions: Systematic Review and Meta-Analysis
Abstract
Background: Prostate cancer is a leading cause of cancer among men. Because screening for prostate cancer is a controversial issue, many experts in the field have defended the use of shared decision making using validated decision aids, which can be presented in different formats (eg, written, multimedia, Web). Recent studies have concluded that decision aids improve knowledge and reduce decisional conflict.
Objective: This meta-analysis aimed to investigate the impact of using Web-based decision aids to support men's prostate cancer screening decisions in comparison with usual care and other formats of decision aids.
Methods: We searched PubMed, CINAHL, PsycINFO, and Cochrane CENTRAL databases up to November 2016. This search identified randomized controlled trials, which assessed Web-based decision aids for men making a prostate cancer screening decision and reported quality of decision-making outcomes. Two reviewers independently screened citations for inclusion criteria, extracted data, and assessed risk of bias. Using a random-effects model, meta-analyses were conducted pooling results using mean differences (MD), standardized mean differences (SMD), and relative risks (RR).
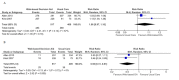
Results: Of 2406 unique citations, 7 randomized controlled trials met the inclusion criteria. For risk of bias, selective outcome reporting and participant/personnel blinding were mostly rated as unclear due to inadequate reporting. Based on seven items, two studies had high risk of bias for one item. Compared to usual care, Web-based decision aids increased knowledge (SMD 0.46; 95% CI 0.18-0.75), reduced decisional conflict (MD -7.07%; 95% CI -9.44 to -4.71), and reduced the practitioner control role in the decision-making process (RR 0.50; 95% CI 0.31-0.81). Web-based decision aids compared to printed decision aids yielded no differences in knowledge, decisional conflict, and participation in decision or screening behaviors. Compared to video decision aids, Web-based decision aids showed lower average knowledge scores (SMD -0.50; 95% CI -0.88 to -0.12) and a slight decrease in prostate-specific antigen screening (RR 1.12; 95% CI 1.01-1.25).
Conclusions: According to this analysis, Web-based decision aids performed similarly to alternative formats (ie, printed, video) for the assessed decision-quality outcomes. The low cost, readiness, availability, and anonymity of the Web can be an advantage for increasing access to decision aids that support prostate cancer screening decisions among men.
Keywords: decision aid; decision making; internet; patient participation; prostate; screening.
©Sofia Baptista, Elvira Teles Sampaio, Bruno Heleno, Luís Filipe Azevedo, Carlos Martins. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 26.06.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





References
-
- Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC, PLCO Project Team Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012 Jan 18;104(2):125–132. doi: 10.1093/jnci/djr500. http://europepmc.org/abstract/MED/22228146 djr500 - DOI - PMC - PubMed
-
- Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L, Lilja H, Denis LJ, Recker F, Paez A, Bangma CH, Carlsson S, Puliti D, Villers A, Rebillard X, Hakama M, Stenman U, Kujala P, Taari K, Aus G, Huber A, van DKTH, van SRHN, de KHJ, Moss SM, Auvinen A. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 Dec 6;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0. http://europepmc.org/abstract/MED/25108889 S0140-6736(14)60525-0 - DOI - PMC - PubMed
-
- Heijnsdijk EAM, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, Kwiatkowski M, Villers A, Páez A, Moss SM, Zappa M, Tammela TLJ, Mäkinen T, Carlsson S, Korfage IJ, Essink-Bot M, Otto SJ, Draisma G, Bangma CH, Roobol MJ, Schröder FH, de KHJ. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012 Aug 16;367(7):595–605. doi: 10.1056/NEJMoa1201637. http://europepmc.org/abstract/MED/22894572 - DOI - PMC - PubMed
-
- Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI, Lane JA, Noble S, Oliver SE, Evans S, Sterne JAC, Holding P, Ben-Shlomo Y, Brindle P, Williams NJ, Hill EM, Ng SY, Toole J, Tazewell MK, Hughes LJ, Davies CF, Thorn JC, Down E, Davey SG, Neal DE, Hamdy FC, Trial Group CAP. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA. 2018 Dec 06;319(9):883–895. doi: 10.1001/jama.2018.0154.2673968 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

