SOX2 regulation by hedgehog signaling controls adult lingual epithelium homeostasis
- PMID: 29945863
- PMCID: PMC6078335
- DOI: 10.1242/dev.164889
SOX2 regulation by hedgehog signaling controls adult lingual epithelium homeostasis
Abstract
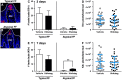
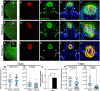
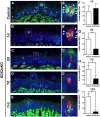
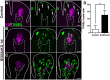
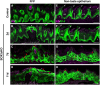
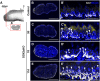
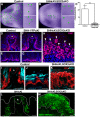
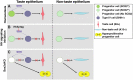
Adult tongue epithelium is continuously renewed from epithelial progenitor cells, a process that requires hedgehog (HH) signaling. In mice, pharmacological inhibition of the HH pathway causes taste bud loss within a few weeks. Previously, we demonstrated that sonic hedgehog (SHH) overexpression in lingual progenitors induces ectopic taste buds with locally increased SOX2 expression, suggesting that taste bud differentiation depends on SOX2 downstream of HH. To test this, we inhibited HH signaling in mice and observed a rapid decline in Sox2 and SOX2-GFP expression in taste epithelium. Upon conditional deletion of Sox2, differentiation of both taste and non-taste epithelial cells was blocked, and progenitor cell number increased. In contrast to basally restricted proliferation in controls, dividing cells were overabundant and spread to suprabasal epithelial layers in mutants. SOX2 loss in progenitors also led non-cell-autonomously to taste cell apoptosis, dramatically shortening taste cell lifespans. Finally, in tongues with conditional Sox2 deletion and SHH overexpression, ectopic and endogenous taste buds were not detectable; instead, progenitor hyperproliferation expanded throughout the lingual epithelium. In summary, we show that SOX2 functions downstream of HH signaling to regulate lingual epithelium homeostasis.
Keywords: Cell differentiation; Mouse; Progenitor proliferation; Smoothened antagonist; Taste bud.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

