Cxcl12a induces snail1b expression to initiate collective migration and sequential Fgf-dependent neuromast formation in the zebrafish posterior lateral line primordium
- PMID: 29945870
- PMCID: PMC6078336
- DOI: 10.1242/dev.162453
Cxcl12a induces snail1b expression to initiate collective migration and sequential Fgf-dependent neuromast formation in the zebrafish posterior lateral line primordium
Abstract
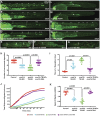
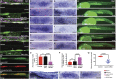
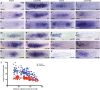
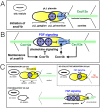
The zebrafish posterior lateral line primordium migrates along a path defined by the chemokine Cxcl12a, periodically depositing neuromasts, to pioneer formation of the zebrafish posterior lateral line system. snail1b, known for its role in promoting cell migration, is expressed in leading cells of the primordium in response to Cxcl12a, whereas its expression in trailing cells is inhibited by Fgf signaling. snail1b knockdown delays initiation of primordium migration. This delay is associated with aberrant expansion of epithelial cell adhesion molecule (epcam) and reduction of cadherin 2 expression in the leading part of the primordium. Co-injection of snail1b morpholino with snail1b mRNA prevents the initial delay in migration and restores normal expression of epcam and cadherin 2 The delay in initiating primordium migration in snail1b morphants is accompanied by a delay in sequential formation of trailing Fgf signaling centers and associated protoneuromasts. This delay is not specifically associated with knockdown of snail1b but also with other manipulations that delay migration of the primordium. These observations reveal an unexpected link between the initiation of collective migration and sequential formation of protoneuromasts in the primordium.
Keywords: Cell adhesion molecule; Collective migration; Cxcl12a; Lateral line primordium; Snail1b; Zebrafish.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Balabanian K., Lagane B., Infantino S., Chow K. Y. C., Harriague J., Moepps B., Arenzana-Seisdedos F., Thelen M. and Bachelerie F. (2005). The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 280, 35760-35766. 10.1074/jbc.M508234200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

