Molecular mechanisms of the preventable causes of cancer in the United States
- PMID: 29945886
- PMCID: PMC6075032
- DOI: 10.1101/gad.314849.118
Molecular mechanisms of the preventable causes of cancer in the United States
Abstract
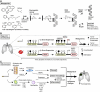
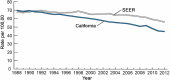
Annually, there are 1.6 million new cases of cancer and nearly 600,000 cancer deaths in the United States alone. The public health burden associated with these numbers has motivated enormous research efforts into understanding the root causes of cancer. These efforts have led to the recognition that between 40% and 45% of cancers are associated with preventable risk factors and, importantly, have identified specific molecular mechanisms by which these exposures modify human physiology to induce or promote cancer. The increasingly refined knowledge of these mechanisms, which we summarize here, emphasizes the need for greater efforts toward primary cancer prevention through mitigation of modifiable risk factors. It also suggests exploitable avenues for improved secondary prevention (which includes the development of therapeutics designed for cancer interception and enhanced techniques for noninvasive screening and early detection) based on detailed knowledge of early neoplastic pathobiology. Such efforts would complement the current emphasis on the development of therapeutic approaches to treat established cancers and are likely to result in far greater gains in reducing morbidity and mortality.
Keywords: cancer mechanisms; cancer prevention; early detection.
© 2018 Golemis et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Adcock IM, Caramori G, Barnes PJ. 2011. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration 81: 265–284. - PubMed
-
- Alam M, Ratner D. 2001. Cutaneous squamous-cell carcinoma. N Engl J Med 344: 975–983. - PubMed
-
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, et al. 1996. α-Tocopherol and β-carotene supplements and lung cancer incidence in the alpha-tocopherol, β-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 88: 1560–1570. - PubMed
-
- Albini A, DeCensi A, Cavalli F, Costa A. 2016. Cancer prevention and interception: a new era for chemopreventive approaches. Clin Cancer Res 22: 4322–4327. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
