Dopamine Transporter Dynamics of N-Substituted Benztropine Analogs with Atypical Behavioral Effects
- PMID: 29945932
- PMCID: PMC6102189
- DOI: 10.1124/jpet.118.250498
Dopamine Transporter Dynamics of N-Substituted Benztropine Analogs with Atypical Behavioral Effects
Abstract
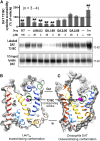
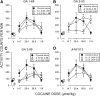
Atypical dopamine transporter (DAT) inhibitors, despite high DAT affinity, do not produce the psychomotor stimulant and abuse profile of standard DAT inhibitors such as cocaine. Proposed contributing features for those differences include off-target actions, slow onsets of action, and ligand bias regarding DAT conformation. Several 3α-(4',4''-difluoro-diphenylmethoxy)tropanes were examined, including those with the following substitutions: N-(indole-3''-ethyl)- (GA1-69), N-(R)-2''-amino-3''-methyl-n-butyl- (GA2-50), N-2''aminoethyl- (GA2-99), and N-(cyclopropylmethyl)- (JHW013). These compounds were previously reported to have rapid onset of behavioral effects and were presently evaluated pharmacologically alone or in combination with cocaine. DAT conformational mode was assessed by substituted-cysteine accessibility and molecular dynamics (MD) simulations. As determined by substituted-cysteine alkylation, all BZT analogs except GA2-99 showed bias for a cytoplasmic-facing DAT conformation, whereas cocaine stabilized the extracellular-facing conformation. MD simulations suggested that several analog-DAT complexes formed stable R85-D476 "outer gate" bonds that close the DAT to extracellular space. GA2-99 diverged from this pattern, yet had effects similar to those of other atypical DAT inhibitors. Apparent DAT association rates of the BZT analogs in vivo were slower than that for cocaine. None of the compounds was self-administered or stimulated locomotion, and each blocked those effects of cocaine. The present findings provide more detail on ligand-induced DAT conformations and indicate that aspects of DAT conformation other than "open" versus "closed" may facilitate predictions of the actions of DAT inhibitors and may promote rational design of potential treatments for psychomotor-stimulant abuse.
U.S. Government work not protected by U.S. copyright.
Figures







References
-
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. (1997) Novel N-substituted 3 α-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40:4329–4339. - PubMed
-
- Bergman J, Madras BK, Johnson SE, Spealman RD. (1989) Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther 251:150–155. - PubMed
-
- Corwin RL, Woolverton WL, Schuster CR. (1990) Effects of cholecystokinin, d-amphetamine and fenfluramine in rats trained to discriminate 3 from 22 hr of food deprivation. J Pharmacol Exp Ther 253:720–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

