The use of defibrotide in blood and marrow transplantation
- PMID: 29945939
- PMCID: PMC6020812
- DOI: 10.1182/bloodadvances.2017008375
The use of defibrotide in blood and marrow transplantation
Erratum in
-
Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018;2(12):1495-1509.Blood Adv. 2018 Aug 14;2(15):1853. doi: 10.1182/bloodadvances.2018023168. Blood Adv. 2018. PMID: 30061309 Free PMC article. No abstract available.
Abstract
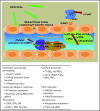
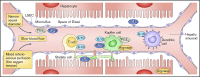
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially life-threatening complication of conditioning during hematopoietic stem cell transplantation (HSCT) or chemotherapy without HSCT, with a historically reported mean incidence of 13.7% post-HSCT. Typical symptoms of VOD/SOS may include hyperbilirubinemia, painful hepatomegaly, weight gain, and ascites. Defibrotide, a polydisperse mixture of predominantly single-stranded polydeoxyribonucleotides, is currently the only therapy approved to treat hepatic VOD/SOS with pulmonary/renal dysfunction (ie, multiorgan dysfunction/multiorgan failure [MOD/MOF]) following HSCT in the United States and to treat severe hepatic VOD/SOS post-HSCT in the European Union. In preclinical and human studies, defibrotide has demonstrated profibrinolytic, antithrombotic, anti-inflammatory, and angio-protective actions, thus promoting an anticoagulant phenotype of the endothelium that protects and stabilizes the function of endothelial cells. In a phase 3, historically controlled, multicenter trial in adults and children with VOD/SOS and MOD/MOF (defibrotide: n = 102; controls treated before defibrotide availability: n = 32), defibrotide resulted in significantly greater day +100 survival following HSCT (38.2%) vs controls (25.0%; propensity analysis-estimated between-group difference: 23%; P = .0109). The most common adverse events (AEs) were hypotension and diarrhea; rates of common hemorrhagic AEs were similar in the defibrotide and historical control group (64% and 75%, respectively). In a phase 3 prophylaxis trial, defibrotide was found to lower incidence of VOD/SOS in children (not an approved indication) and reduce the incidence of graft-versus-host disease. This review describes the development and clinical applications of defibrotide, focusing on its on-label use in patients with VOD/SOS and MOD/MOF after HSCT.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.G.R. has served on advisory committees and received research funding from Jazz Pharmaceuticals. E.C. has served on advisory boards and the speakers bureau for, received research funding from, and provided expert testimony for Gentium. M.I. was formerly an employee of Gentium. B.N. was formerly an employee of Jazz Pharmaceuticals; in the course of employment, he received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc.
Figures

References
-
- Gentium SA. Executive informational overview. http://cdn2.hubspot.net/hub/150154/file-18094943-pdf/docs/gent_eio_06-26.... Accessed 12 September 2017.
-
- Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vascul Pharmacol. 2013;59(1-2):1-10. - PubMed
-
- Niada R, Mantovani M, Prino G, et al. Antithrombotic activity of a polydeoxyribonucleotidic substance extracted from mammalian organs: a possible link with prostacyclin. Thromb Res. 1981;23(3):233-246. - PubMed
-
- Falanga A, Vignoli A, Marchetti M, Barbui T. Defibrotide reduces procoagulant activity and increases fibrinolytic properties of endothelial cells. Leukemia. 2003;17(8):1636-1642. - PubMed
-
- Echart CL, Graziadio B, Somaini S, et al. The fibrinolytic mechanism of defibrotide: effect of defibrotide on plasmin activity. Blood Coagul Fibrinolysis. 2009;20(8):627-634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

