Ca2+-mitochondria axis drives cell division in hematopoietic stem cells
- PMID: 29946000
- PMCID: PMC6080917
- DOI: 10.1084/jem.20180421
Ca2+-mitochondria axis drives cell division in hematopoietic stem cells
Abstract
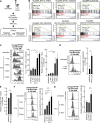
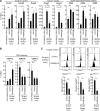
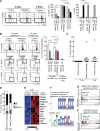
Most of the hematopoietic stem cells (HSCs) within the bone marrow (BM) show quiescent state with a low mitochondrial membrane potential (ΔΨm). In contrast, upon stress hematopoiesis, HSCs actively start to divide. However, the underlying mechanism for the initiation of HSC division still remains unclear. To elucidate the mechanism underlying the transition of cell cycle state in HSCs, we analyzed the change of mitochondria in HSCs after BM suppression induced by 5-fluoruracil (5-FU). We found that HSCs initiate cell division after exhibiting enhanced ΔΨm as a result of increased intracellular Ca2+ level. Although further activation of Ca2+-mitochondria pathway led to loss of HSCs after cell division, the appropriate suppression of intracellular Ca2+ level by exogenous adenosine or Nifedipine, a Ca2+ channel blocker, prolonged cell division interval in HSCs, and simultaneously achieved both cell division and HSC maintenance. Collectively, our results indicate that the Ca2+-mitochondria pathway induces HSC division critically to determine HSC cell fate.
© 2018 Umemoto et al.
Figures





Comment in
-
Ca2+tapulting HSCs into action.J Exp Med. 2018 Aug 6;215(8):1971-1973. doi: 10.1084/jem.20181097. Epub 2018 Jul 12. J Exp Med. 2018. PMID: 30002075 Free PMC article.
References
-
- Bidaux G., Borowiec A.S., Gordienko D., Beck B., Shapovalov G.G., Lemonnier L., Flourakis M., Vandenberghe M., Slomianny C., Dewailly E., et al. 2015. Epidermal TRPM8 channel isoform controls the balance between keratinocyte proliferation and differentiation in a cold-dependent manner. Proc. Natl. Acad. Sci. USA. 112:E3345–E3354. 10.1073/pnas.1423357112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

