Endogenous Protection from Ischemic Brain Injury by Preconditioned Monocytes
- PMID: 29946039
- PMCID: PMC6067076
- DOI: 10.1523/JNEUROSCI.0324-18.2018
Endogenous Protection from Ischemic Brain Injury by Preconditioned Monocytes
Abstract
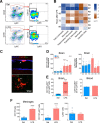
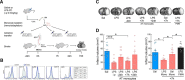
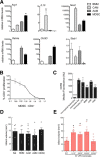
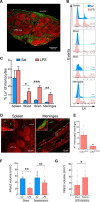
Exposure to low-dose lipopolysaccharide (LPS) before cerebral ischemia is neuroprotective in stroke models, a phenomenon termed preconditioning (PC). Although it is well established that LPS-PC induces central and peripheral immune responses, the cellular mechanisms modulating ischemic injury remain unclear. Here, we investigated the role of immune cells in the brain protection afforded by PC and tested whether monocytes may be reprogrammed by ex vivo LPS exposure, thus modulating inflammatory injury after cerebral ischemia in male mice. We found that systemic injection of low-dose LPS induces a Ly6Chi monocyte response that protects the brain after transient middle cerebral artery occlusion (MCAO) in mice. Remarkably, adoptive transfer of monocytes isolated from preconditioned mice into naive mice 7 h after transient MCAO reduced brain injury. Gene expression and functional studies showed that IL-10, inducible nitric oxide synthase, and CCR2 in monocytes are essential for neuroprotection. This protective activity was elicited even if mouse or human monocytes were exposed ex vivo to LPS and then injected into male mice after stroke. Cell-tracking studies showed that protective monocytes are mobilized from the spleen and reach the brain and meninges, where they suppress postischemic inflammation and neutrophil influx into the brain parenchyma. Our findings unveil a previously unrecognized subpopulation of splenic monocytes capable of protecting the brain with an extended therapeutic window and provide the rationale for cell therapies based on the delivery of autologous or allogeneic protective monocytes in patients after ischemic stroke.SIGNIFICANCE STATEMENT Inflammation is a key component of the pathophysiology of the brain in stroke, a leading cause of death and disability with limited therapeutic options. Here, we investigate endogenous mechanisms of protection against cerebral ischemia. Using lipopolysaccharide (LPS) preconditioning (PC) as an approach to induce ischemic tolerance in mice, we found generation of neuroprotective monocytes within the spleen, from which they traffic to the brain and meninges, suppressing postischemic inflammation. Importantly, systemic LPS-PC can be mimicked by adoptive transfer of in vitro-preconditioned mouse or human monocytes at translational relevant time points after stroke. This model of neuroprotection may facilitate clinical efforts to increase the efficacy of BM mononuclear cell treatments in acute neurological diseases such as cerebral ischemia.
Keywords: adoptive transfer; cerebral ischemia; lipopolysaccharide; monocytes; neuroprotection.
Copyright © 2018 the authors 0270-6474/18/386722-15$15.00/0.
Figures


Similar articles
-
Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice.Stroke. 2004 Nov;35(11):2576-81. doi: 10.1161/01.STR.0000143450.04438.ae. Epub 2004 Sep 16. Stroke. 2004. PMID: 15375302
-
LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury.J Neuroinflammation. 2011 Oct 14;8:140. doi: 10.1186/1742-2094-8-140. J Neuroinflammation. 2011. PMID: 21999375 Free PMC article.
-
Lipopolysaccharide preconditioning increased the level of regulatory B cells in the spleen after acute ischaemia/reperfusion in mice.Brain Res. 2018 Dec 15;1701:46-57. doi: 10.1016/j.brainres.2018.05.036. Epub 2018 May 24. Brain Res. 2018. PMID: 29803621
-
Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research.J Mol Cell Cardiol. 2001 Nov;33(11):1897-918. doi: 10.1006/jmcc.2001.1462. J Mol Cell Cardiol. 2001. PMID: 11708836 Review.
-
Role of Circulating Immune Cells in Stroke and Preconditioning-Induced Protection.Acta Neurochir Suppl. 2016;121:39-44. doi: 10.1007/978-3-319-18497-5_7. Acta Neurochir Suppl. 2016. PMID: 26463920 Free PMC article. Review.
Cited by
-
Monocytes maintain central nervous system homeostasis following helminth-induced inflammation.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2201645119. doi: 10.1073/pnas.2201645119. Epub 2022 Sep 7. Proc Natl Acad Sci U S A. 2022. PMID: 36070344 Free PMC article.
-
The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials.Int J Mol Sci. 2022 Mar 23;23(7):3485. doi: 10.3390/ijms23073485. Int J Mol Sci. 2022. PMID: 35408846 Free PMC article. Review.
-
The "Dialogue" Between Central and Peripheral Immunity After Ischemic Stroke: Focus on Spleen.Front Immunol. 2021 Dec 16;12:792522. doi: 10.3389/fimmu.2021.792522. eCollection 2021. Front Immunol. 2021. PMID: 34975893 Free PMC article. Review.
-
Preconditioning with CpG-ODN1826 reduces ischemic brain injury in young male mice: a replication study.Cond Med. 2019;2(4):178-184. Cond Med. 2019. PMID: 32510041 Free PMC article.
-
Microglial/Macrophage polarization and function in brain injury and repair after stroke.CNS Neurosci Ther. 2021 May;27(5):515-527. doi: 10.1111/cns.13620. Epub 2021 Mar 1. CNS Neurosci Ther. 2021. PMID: 33650313 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
