Host lipidome analysis during rhinovirus replication in HBECs identifies potential therapeutic targets
- PMID: 29946055
- PMCID: PMC6121942
- DOI: 10.1194/jlr.M085910
Host lipidome analysis during rhinovirus replication in HBECs identifies potential therapeutic targets
Abstract
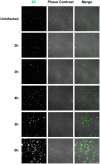
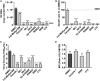
In patients with asthma or chronic obstructive pulmonary disease, rhinovirus (RV) infections can provoke acute worsening of disease, and limited treatment options exist. Viral replication in the host cell induces significant remodeling of intracellular membranes, but few studies have explored this mechanistically or as a therapeutic opportunity. We performed unbiased lipidomic analysis on human bronchial epithelial cells infected over a 6 h period with the RV-A1b strain of RV to determine changes in 493 distinct lipid species. Through pathway and network analysis, we identified temporal changes in the apparent activities of a number of lipid metabolizing and signaling enzymes. In particular, analysis highlighted FA synthesis and ceramide metabolism as potential anti-rhinoviral targets. To validate the importance of these enzymes in viral replication, we explored the effects of commercially available enzyme inhibitors upon RV-A1b infection and replication. Ceranib-1, D609, and C75 were the most potent inhibitors, which confirmed that FAS and ceramidase are potential inhibitory targets in rhinoviral infections. More broadly, this study demonstrates the potential of lipidomics and pathway analysis to identify novel targets to treat human disorders.
Keywords: ceramidase; fatty acid synthase; human bronchial epithelial cells; lipidomics.
Copyright © 2018 Nguyen et al.
Conflict of interest statement
Additional support was provided by the National Heart and Lung Institute (student funding to A.G.) and Medical Research Council Centre Grant G10000758 (to the Medical Research Council and Asthma United Kingdom Centre in Allergic Mechanisms of Asthma of which A.M., A.G., D.S., and R.S. were members).
Figures





References
-
- Spickler C., Lippens J., Laberge M. K., Desmeules S., Bellavance E., Garneau M., Guo T., Hucke O., Leyssen P., Neyts J., et al. . 2013. Phosphatidylinositol 4-kinase III beta is essential for replication of human rhinovirus and its inhibition causes a lethal phenotype in vivo. Antimicrob. Agents Chemother. 57: 3358–3368. - PMC - PubMed
-
- Albulescu L., Strating J. R., Thibaut H. J., van der Linden L., Shair M. D., Neyts J., and van Kuppeveld F. J.. 2015. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antiviral Res. 117: 110–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/P013384/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/M004821/1/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/000C0415/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/000C0432 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_PC_15028/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

