Pulmonary Arterial Hypertension Affects the Rat Gut Microbiome
- PMID: 29946072
- PMCID: PMC6018770
- DOI: 10.1038/s41598-018-27682-w
Pulmonary Arterial Hypertension Affects the Rat Gut Microbiome
Abstract
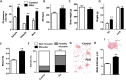
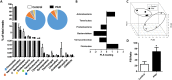
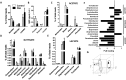
We have analysed whether pulmonary arterial hypertension (PAH) alters the rat faecal microbiota. Wistar rats were injected with the VEGF receptor antagonist SU5416 (20 mg/kg s.c.) and followed for 2 weeks kept in hypoxia (10% O2, PAH) or injected with vehicle and kept in normoxia (controls). Faecal samples were obtained and microbiome composition was determined by 16S rRNA gene sequencing and bioinformatic analysis. No effect of PAH on the global microbiome was found (α- or β-diversity). However, PAH-exposed rats showed gut dysbiosis as indicated by a taxonomy-based analysis. Specifically, PAH rats had a three-fold increase in Firmicutes-to-Bacteroidetes ratio. Within the Firmicutes phylum, there were no large changes in the relative abundance of the bacterial families in PAH. Among Bacteroidetes, all families were less abundant in PAH. A clear separation was observed between the control and PAH clusters based on short chain fatty acid producing bacterial genera. Moreover, acetate was reduced in the serum of PAH rats. In conclusion, faecal microbiota composition is altered as a result of PAH. This misbalanced bacterial ecosystem might in turn play a pathophysiological role in PAH by altering the immunologic, hormonal and metabolic homeostasis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Galie N, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

