Dissociation of C-Reactive Protein Localizes and Amplifies Inflammation: Evidence for a Direct Biological Role of C-Reactive Protein and Its Conformational Changes
- PMID: 29946323
- PMCID: PMC6005900
- DOI: 10.3389/fimmu.2018.01351
Dissociation of C-Reactive Protein Localizes and Amplifies Inflammation: Evidence for a Direct Biological Role of C-Reactive Protein and Its Conformational Changes
Abstract
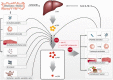
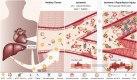
C-reactive protein (CRP) is a member of the pentraxin superfamily that is widely recognized as a marker of inflammatory reactions and cardiovascular risk in humans. Recently, a growing body of data is emerging, which demonstrates that CRP is not only a marker of inflammation but also acts as a direct mediator of inflammatory reactions and the innate immune response. Here, we critically review the various lines of evidence supporting the concept of a pro-inflammatory "CRP system." The CRP system consists of a functionally inert circulating pentameric form (pCRP), which is transformed to its highly pro-inflammatory structural isoforms, pCRP* and ultimately to monomeric CRP (mCRP). While retaining an overall pentameric structure, pCRP* is structurally more relaxed than pCRP, thus exposing neoepitopes important for immune activation and complement fixation. Thereby, pCRP* shares its pro-inflammatory properties with the fully dissociated structural isoform mCRP. The dissociation of pCRP into its pro-inflammatory structural isoforms and thus activation of the CRP system occur on necrotic, apoptotic, and ischemic cells, regular β-sheet structures such as β-amyloid, the membranes of activated cells (e.g., platelets, monocytes, and endothelial cells), and/or the surface of microparticles, the latter by binding to phosphocholine. Both pCRP* and mCRP can cause activation of platelets, leukocytes, endothelial cells, and complement. The localization and deposition of these pro-inflammatory structural isoforms of CRP in inflamed tissue appear to be important mediators for a range of clinical conditions, including ischemia/reperfusion (I/R) injury of various organs, cardiovascular disease, transplant rejection, Alzheimer's disease, and age-related macular degeneration. These findings provide the impetus to tackle the vexing problem of innate immunity response by targeting CRP. Understanding the "activation process" of CRP will also likely allow the development of novel anti-inflammatory drugs, thereby providing potential new immunomodulatory therapeutics in a broad range of inflammatory diseases.
Keywords: Alzheimer disease; C-reactive protein; cardiovascular diseases; inflammation; ischemia/reperfusion.
Figures
References
-
- Abernethy TJ, Avery OT. The occurrence during acute infections of a protein not normally present in the blood: I. Distribution of the reactive protein in patients’ sera and the effect of calcium on the flocculation reaction with C polysaccharide of pneumococcus. J Exp Med (1941) 73:173–82.10.1084/jem.73.2.173 - DOI - PMC - PubMed
-
- Volanakis JE, Kaplan MH. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes: requirement for human C1q. J Immunol (1974) 113:9–17. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

